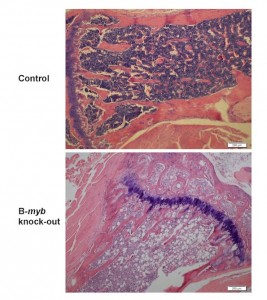
Loss of B-myb expression results in a loss of cellularity as evidenced in hematoxylin and eosin (H&E) stained paraffin-embedded bone marrow sections.
Role of MYB Family Genes in Development and Cancer
Is a tightly regulated process by which hematopoietic stem cells (HSCs) give rise to mature cells. The balance between the propensity of HSCs to remain quiescent, to divide and generate more HSCs (self-renewal), or to divide and give rise to mature cells (differentiation) is essential for the long-term maintenance of blood cell formation. Mechanisms underlying cell fate decisions of HSCs are not completely understood. The expression levels and temporal expression patterns of transcription factors, including member of the MYB family, play key roles in HSC function and in downstream lineage choices.
The MYB family has three members, A-myb (MYBL1), B-myb (MYBL2) and c-myb (MYB). Using conditional knock-out mouse models, our studies showed that loss of c-myb expression results in a block in thymocyte development and in a critical depletion of the hematopoietic stem cell (HSC) pool, which leads to dramatic reductions of mature cells of various lineages including neutrophilic, monocytic, B lymphoid, erythroid, and, unexpectedly, megakaryocytic cells in the bone marrow (BM). Serial BM transplantation into lethally irradiated recipient mice indicates that c-myb is essential for HSC self-renewal, a phenotype that results from a reduced proliferative capacity and aberrant, accelerated differentiation of HSCs. C-myb also plays a role in myelopoiesis and results in a bias towards monocytic development.
Conditional inactivation of B-myb in vivo also results in depletion of the HSC pool, and leads to profound reductions in mature lymphoid, erythroid and myeloid cells. This defect is autonomous to the bone marrow and is first evident in stem cells, which accumulate in the S and G2/M phases. B-myb inactivation also causes defects in the myeloid progenitor compartment, consisting of depletion of common myeloid and megakaryocyte-erythroid progenitors but relative sparing of granulocyte-macrophage progenitors. Microarray studies indicate that B-myb null LKS+ cells differentially express genes that direct myeloid lineage development and commitment, suggesting that B-myb is a key player in controlling cell fate. Collectively, these studies demonstrate that B-myb is essential for HSC and progenitor maintenance and survival during hematopoiesis.
Unlike c- and B-myb knock-out mice, which die in utero, systemic disruption of the A-myb locus causes a completely different phenotype. Mice homozygous for germline deletion of A-myb develop to term but show defects in growth after birth and male infertility due to a block in spermatogenesis. Morphological examination of the testes of A-myb-/- males revealed that the germ cells enter meiotic prophase and arrest at pachytene. In adult homozygous null A-myb female mice, the breast epithelial compartment showed underdevelopment of breast tissue following pregnancy and an inability to nurse newborn pups. These results demonstrate that A-myb plays a critical role in spermatogenesis and mammary gland development.
We are currently investigating the mechanisms that underlie these phenotypes and the role(s) that these genes play in diseases that result in aberrant proliferation.
