GoCAR:
“Genomics of Chronic Renal Allograft Rejection,” (GoCAR), which aims to investigate the mechanisms leading to the development of transplant glomerulopathy, chronic arteriopathy funded through the NIH/NIAID Genomics Consortium in Transplantation. The study examines the contribution of the direct and indirect pathways of allorecognition, and the development of de novo donor specific antibodies to this pathologic finding in a large prospective group of renal transplant recipients. The studies examines gene expression profiles associated with the development of chronic rejection using microarray on protocol biopsies performed over two years following transplantation. In addition, a larger cohort of donor and recipient pairs are being enrolled to identify polymorphic variants of specific immunological genes which confer susceptibility to chronic rejection and the development of donor specific antibodies
Patients have been enrolled in 5 clinical sites including Mount Sinai, Westmead Hospital Sydney, University Wisconsin – Madison Medical Center, University of Michigan Medical Center, Ann Arbor, and Northwestern Medical Center. The findings of this study will lead to important insights into the pathologic mechanisms mediating chronic allograft loss. Initial finding suggest that differential gene expression on normal 3 month protocol biopsies may be potentially used to immunologically stratify transplant recipients.
SHROOM3 in kidney fibrosis:
Chronic kidney disease or CKD is a major public health problem. Progressive CKD requires dialysis or transplantation and is a recognized risk factor for death from cardiovascular disease. Current treatment for progressive CKD is limited. Thus, many with CKD will progress to dialysis or die at an increased rate from vascular disease. Kidney transplantation is the preferred treatment for progressive CKD; however, long term survival of transplanted kidneys is still out of reach due to CKD that recurs in them. When kidneys fail from any cause, a process called fibrosis (scarring) is universally encountered in them.
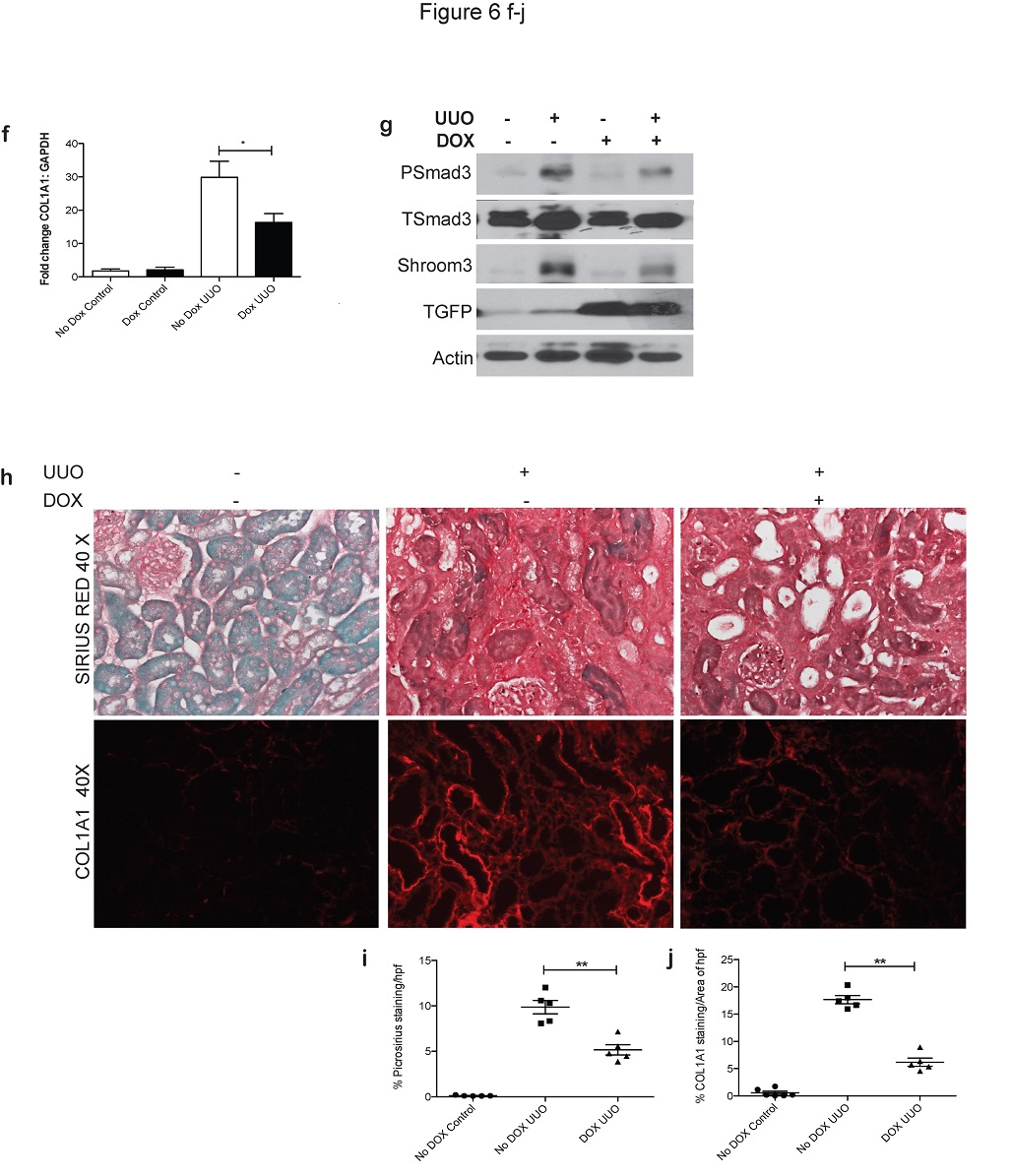
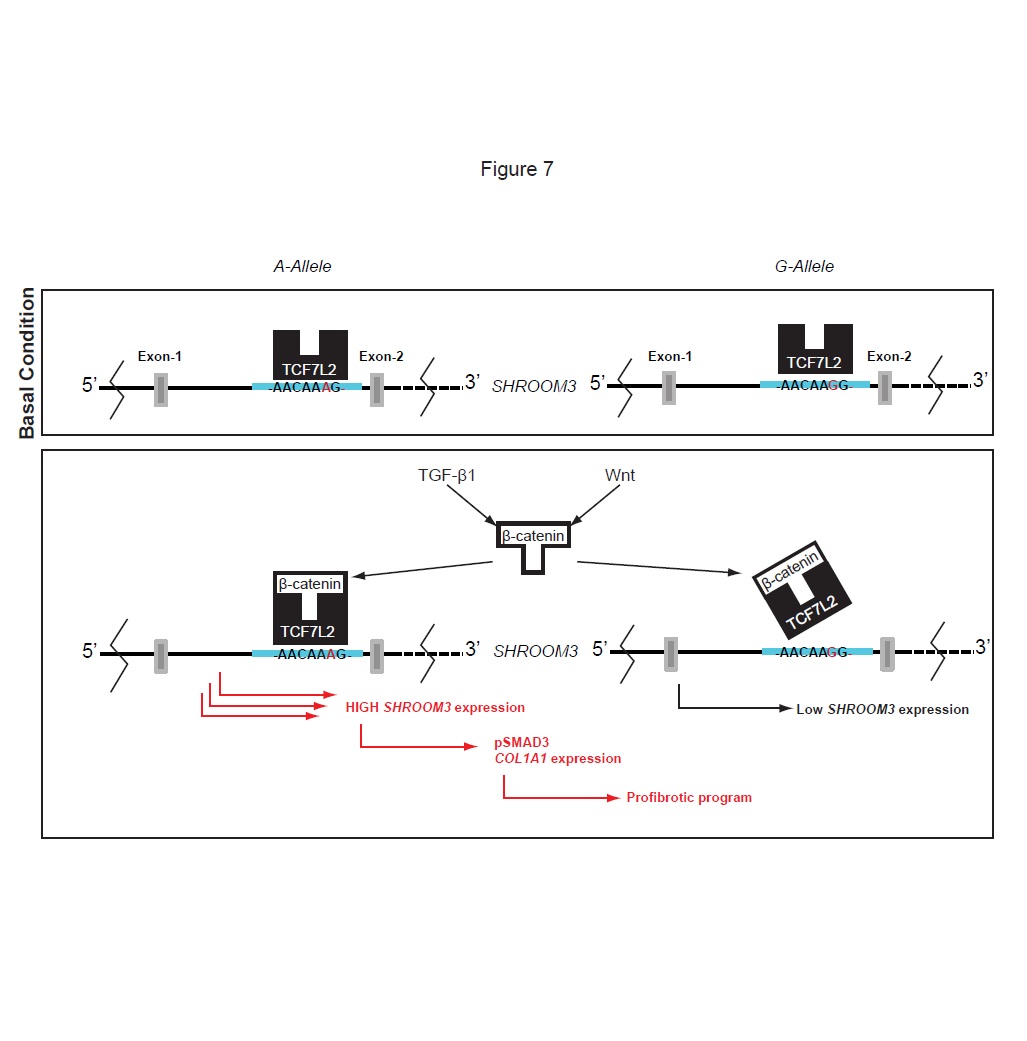
We identified that Shroom3, previously shown to be as a risk factor for CKD, may be a key common link for kidney fibrosis from all causes, including in transplants.
Our initial work showed that a commonly encountered Shroom3 mutation could identify kidney transplants that would develop more fibrosis, and outlined the broad pathway by which this mutation and gene affect fibrosis (See figures).
(1) We use in vitro techniques to understand the exact mechanism by which Shroom3 interacts and promotes fibrosis. Our initial data suggests interaction with TGF-beta and WNT- pathways – both of which are centrally involved in renal fibrosis.
(3) Using mouse models that we have engineered where Shroom3 can be silenced, we study kidney fibrosis in mice with-and-without Shroom3 silencing by performing experiments to induce fibrosis as well as kidney transplant experiments. In the lab, we regularly perform mouse kidney surgeries, induce diabetes for studying diabetic kidney fibrosis and transplants.
(3)We also are working to confirm our findings in a separate patient-group.
In this way we are combining data that emerged from actual human studies, in vitro cellular techniques and genetically engineered mice to understand how this commonly encountered genetic mutation predisposes to progressive CKD, as well as kidney transplant fibrosis. This work is significant because not only will we be able to predict beforehand those kidneys that will not do as well once transplanted, but our experiments can also lead to the development of specific molecules that could affect Shroom3, and therefore reduce kidney fibrosis from most causes.
Our initial work has been published in the JCI 2015 (http://www.jci.org/articles/view/76902) and was highlighted recently in the JCI’s “scientific show-stopper” segment (http://www.jci.org/posts/232).
Please click on the images below for a closer view: