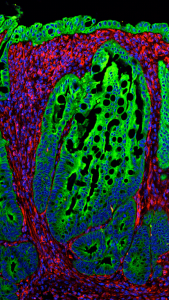
Inflammation and cancer. Our lab has a longstanding interest in the connection between inflammation and cancer. We have contributed to the identification of two viral-induced chemokine receptor oncogenes, and have studied for many years the influence of the inflammation in tumor development. In recent years we have focused our research on the interplay between growth factors such as EGFR and immune molecules such as TLR4 and CX3CL1 in cancer. In a recent study we examined the mechanisms dictating the site-specific development of neoplasms in the intestine, using an animal model of serrated polyps developed in our lab. We showed that the geographic localization of the polyps was dependent on a host-specific, site-specific microbiota. We demonstrated that the presence of the microbiota within the tumors triggered a significant inflammatory response that included recruitment of neutrophils to the polyp. Blockade of neutrophil recruitment reduced the number and size of the polyps, suggesting an important role for inflammation in the process. These results strongly implicate the microbiota as a major factor contributing to development of this specific kind of neoplasms, in addition to the well-established somatic mutations in BRAF, and K-Ras.
a. Bongers G, Muniz LR, Pacer ME, Iuga AC, Thirunarayanan N, Slinger E, Smit MJ, Reddy EP, Mayer L, Furtado GC, Harpaz N, Lira SA. A role for the epidermal growth factor receptor signaling in development of intestinal serrated polyps in mice and humans. Gastroenterology. 2012 Sep;143(3):730-40.
b. Tardáguila M, Mira E, García-Cabezas MA, Feijoo AM, Quintela-Fandino M, Azcoitia I, Lira SA, Mañes S. CX3CL1 promotes breast cancer via transactivation of the EGF pathway. Cancer Res. 2013 Jul 15;73(14):4461-73.
c. Santaolalla R, Sussman DA, Ruiz JR, Davies JM, Pastorini C, España CL, Sotolongo J, Burlingame O, Bejarano PA, Philip S, Ahmed MM, Ko J, Dirisina R, Barrett TA, Shang L, Lira SA, Fukata M, Abreu MT. TLR4 activates the β-catenin pathway to cause intestinal neoplasia. PLoS One. 2013;8(5):e63298.
d. Bongers G, Pacer ME, Geraldino TH, Chen L, He Z, Hashimoto D, Furtado GC, Ochando J, Kelley KA, Clemente JC, Merad M, van Bakel H, Lira SA. Interplay of host microbiota, genetic perturbations, and inflammation promotes local development of intestinal neoplasms in mice. J Exp Med. 2014 Mar 10;211(3):457-72.
The biology of IL-23 in newborn and adult mice Studies done in our lab were the first to show that IL-23 expression promotes inflammation in vivo. Subsequently, we collaborated with Dan Cua and Jon Sedgwick’s labs to show that many of the inflammatory phenotypes previously attributed to IL-12 were in fact driven by IL-23. 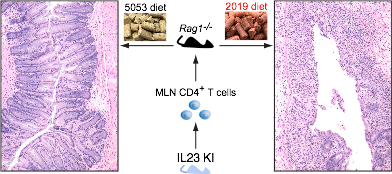 More recently we have studied the contribution of IL-23 to gut immunity and showed that increased expression of IL-23 in the neonatal intestine leads to a dramatic expansion of the innate lymphoid cell compartment, resulting in disruption of the epithelium and neonatal death. Recent work describes the generation of a novel model system to study IL-23 biology in adult mice. IL-23 expression promotes development of disease when expressed by CX3CR1+ cells. Disease induction is dependent on the microbiota and it is mediated by CD4 T cells. Interestingly, flares of disease can be induced by switching the diet given to the mice, indicating a critical role for an environmental factor (diet) in disease initiation and disease flares.
More recently we have studied the contribution of IL-23 to gut immunity and showed that increased expression of IL-23 in the neonatal intestine leads to a dramatic expansion of the innate lymphoid cell compartment, resulting in disruption of the epithelium and neonatal death. Recent work describes the generation of a novel model system to study IL-23 biology in adult mice. IL-23 expression promotes development of disease when expressed by CX3CR1+ cells. Disease induction is dependent on the microbiota and it is mediated by CD4 T cells. Interestingly, flares of disease can be induced by switching the diet given to the mice, indicating a critical role for an environmental factor (diet) in disease initiation and disease flares.
a. Wiekowski MT, Leach MW, Evans EW, Sullivan L, Chen SC, Vassileva G, Bazan JF, Gorman DM, Kastelein RA, Narula S, Lira SA. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol. 2001 Jun 15;166(12):7563-70..
b. Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003 Feb 13;421(6924):744-8.
c. Chen L, He Z, Slinger E, Bongers G, Lapenda TL, Pacer ME, Jiao J, Beltrao MF, Soto AJ, Harpaz N, Gordon RE, Ochando JC, Oukka M, Iuga AC, Chensue SW, Blander JM, Furtado GC, Lira SA. IL-23 activates innate lymphoid cells to promote neonatal intestinal pathology. Mucosal Immunol. 2015 Mar;8(2):390-402.
d. ChenL, He Z, Iuga AC, Martins Filho SN, Faith JJ, Clemente JC, Deshpande M, Jayaprakash A, Colombel JF, Lafaille JJ, Sachidanandam R, Furtado GC, LiraSA. Diet modifies colonic microbiota and CD4+ T cell repertoire to induce flares of colitis in mice with myeloid-cell expression of Interleukin 23. Gastroenterology (2018) doi: 10.1053/j.gastro.2018.06.034. [Epub ahead of print]
Chemokines and chemokine receptors At the beginning of the 90’s little was known about the biological activities of the recently described group of molecules known as chemokines. Using genetic approaches in mice we showed for the first time that CXC and CC chemokines could promote recruitment of specific leukocyte subsets to specific organs. Our initial studies focused on the CXC chemokine KC (CXCL1). Using tissue-specific promoters we targeted expression of this chemokine to thymus, skin, and the brain, and observed that neutrophils accumulated in the vicinity of the cells expressing KC. These results showed for the first time that chemokines, once secreted by cells in the tissue were sufficient to direct trafficking of target cells from the endothelial lumen into the parenchyma. A set of complementary studies focused on the CC chemokine JE (mCCL2) and showed that its expression in vivo could direct trafficking of monocytes to specific organs such as the brain. To improve the understanding of the mechanism of action of chemokines in vivo our lab develops the first conditional models for chemokine expression in mice (using the tet-on system) and showed that tissue recruitment of neutrophils was dependent on the expression levels of CXCL chemokines. This study provided an explanation for the neutrophil paralysis observed in conditions such as sepsis, in which chemokines are highly elevated in circulation.
We also used loss-of-function approaches to generate and study mouse strains deficient in many chemokine receptors, including CCR6, CCR8, D6 (ACKR2), CX3CR1, CCR11 (ACKR4); and chemokine ligands, including CXCL1, CX3CL1 and CXCL15. These studies revealed a critical role for these molecules in a variety of immune processes beyond cell trafficking, including mucosal cell responses, T helper cell responses, microglial toxicity, cell survival, resolution of inflammation, and autoimmunity. Together these and other studies not listed here have contributed to the understanding of the role of chemokines in homeostasis and disease.
a. Lira SA, Zalamea P, Heinrich JN, Fuentes ME, Carrasco D, Lewin AC, Barton DS, Durham S, Bravo R. Expression of the chemokine N51/KC in the thymus and epidermis of transgenic mice results in marked infiltration of a single class of inflammatory cells. J Exp Med. 1994 Dec 1;180(6):2039-48.
b. Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira SA. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995 Dec 15;155(12):5769-76.
c. Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, Sullivan LM, Smith SR, Greenberg HB, Narula SK, Lipp M, Lira SA. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000 May;12(5):495-503.
d. Chensue SW, Lukacs NW, Yang TY, Shang X, Frait KA, Kunkel SL, Kung T, Wiekowski MT, Hedrick JA, Cook DN, Zingoni A, Narula SK, Zlotnik A, Barrat FJ, O’Garra A, Napolitano M, Lira SA. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J Exp Med. 2001 Mar 5;193(5):573-84.
Mouse engineering – We study many different biological processes using the mouse as a model system. Throughout the years we have generated multiple lines of transgenic and knockout mice. We are currently use CRISPR/Cas technology to generate point mutants, knockouts and knockins in the mouse.