Past Research
Within the brain, a small subset of neurons localized to the hypothalamus is key to controlling energy homeostasis. How these cells control these complex behavioral and metabolic processes, particularly in humans, is unclear. A bottleneck in the field is the lack of availability of human hypothalamic neurons. Dr. Wang decided to address this gap in knowledge after deliberating the risks of this project with Dr. Leibel, and succeeded in generating hypothalamic arcuate nucleus (ARC)-like neurons from human iPSC. This was the first protocol of its kind and earned two first-author publications in JCI (2015) and Curr Protoc Hum Genet (2016). The protocol enabled the field to interrogate structural and molecular mechanisms related to control of human body weight, initially using cells containing hypomorphic mutations in genes that confer highly penetrant obesity. We further applied this tool to study the mechanism of monogenic obesity, including Prohormone convertase 1/3 (PC1/3) deficiency (Stem Cell Reports, 2017), Joubert syndrome (JBST) (Cell Metabolism (2014) and JCI insight (2019)), Prader-Willi Syndrome (JCI, 2017) and Bardet-Biedl syndrome (BBS) (first & co-corresponding author, JCI, 2021). These studies have elucidated the molecular mechanisms of obesity caused by a specific mutation in a cellular context that is independent of systemic effects of excessive fat or other confounders. Our studies using iPSC-derived hypothalamic ARC-like neurons provide a powerful cellular platform for the field to investigate the molecular consequences of mutations in genes that control body weight in the central nervous system in humans.
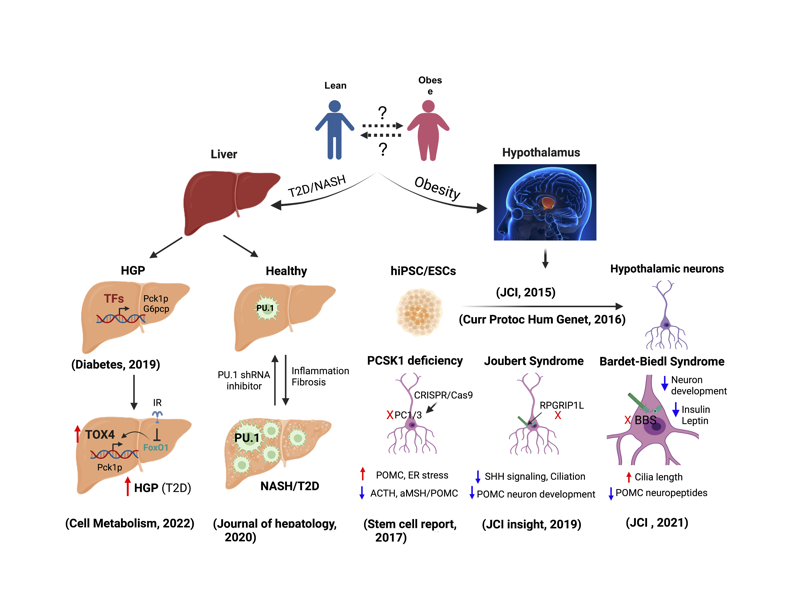
Insulin resistance and T2D are often associated with fatty liver, which may progress to nonalcoholic steatohepatitis (NASH). The underlying mechanisms remain enigmatic. Dr. Wang embarked on a collaboration to identify TFs involved in the development of NAFLD/NASH using a tandem quantitative proteomics strategy. We identified PU.1/SPI1, a master regulator of hematopoiesis, as one of the top TFs induced in livers of DIO and db/db mice and demonstrated a critical role of PU.1 in the pathogenesis of NAFLD/NASH. Pharmacological inhibition of PU.1 improved steatosis, inflammation, fibrosis, glucose intolerance, and dyslipidemia in murine NAFLD/NASH, highlighting its potential therapeutic role (co-first author, J. of Hepatology, 2020).

Ongoing and Future Projects
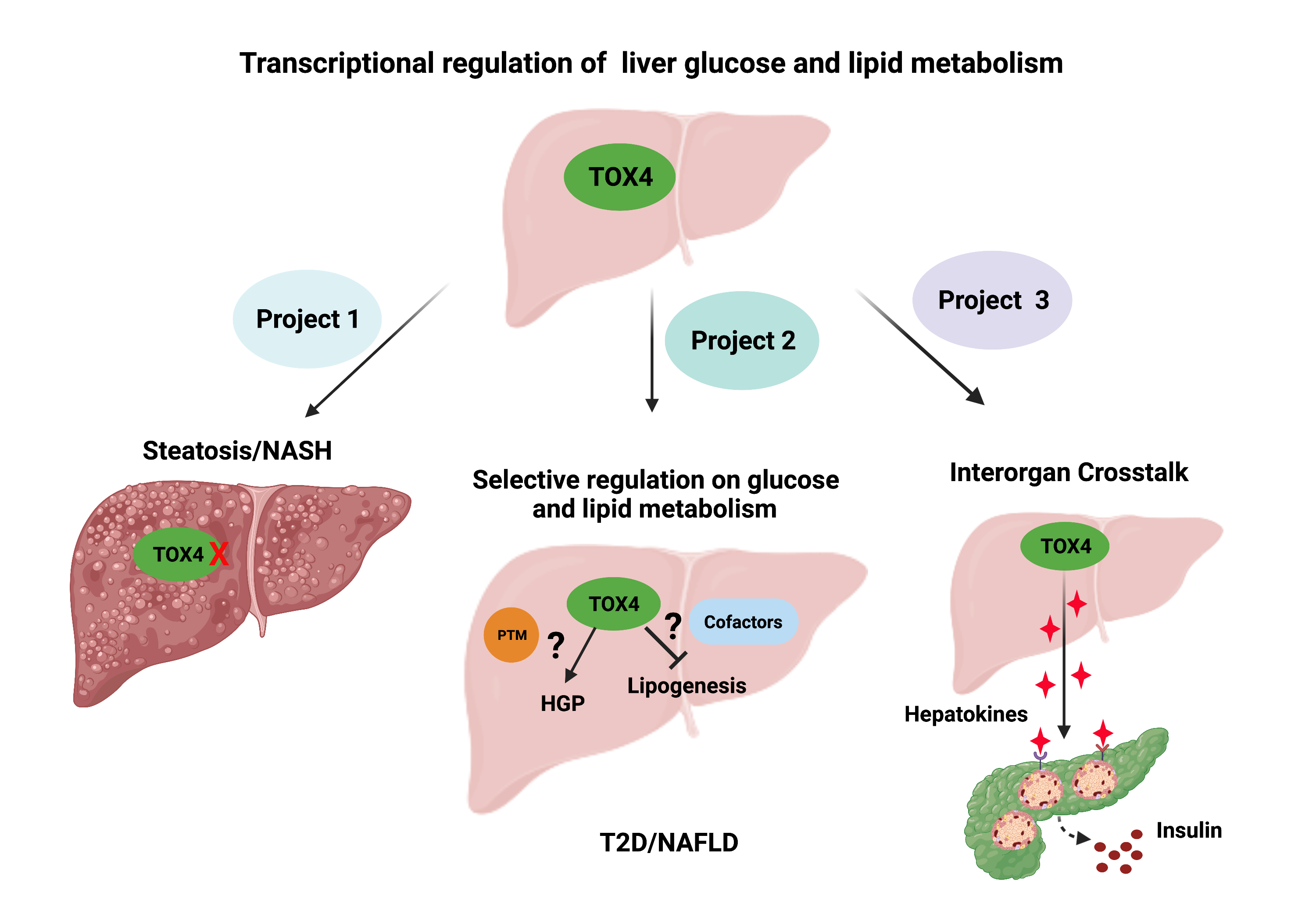
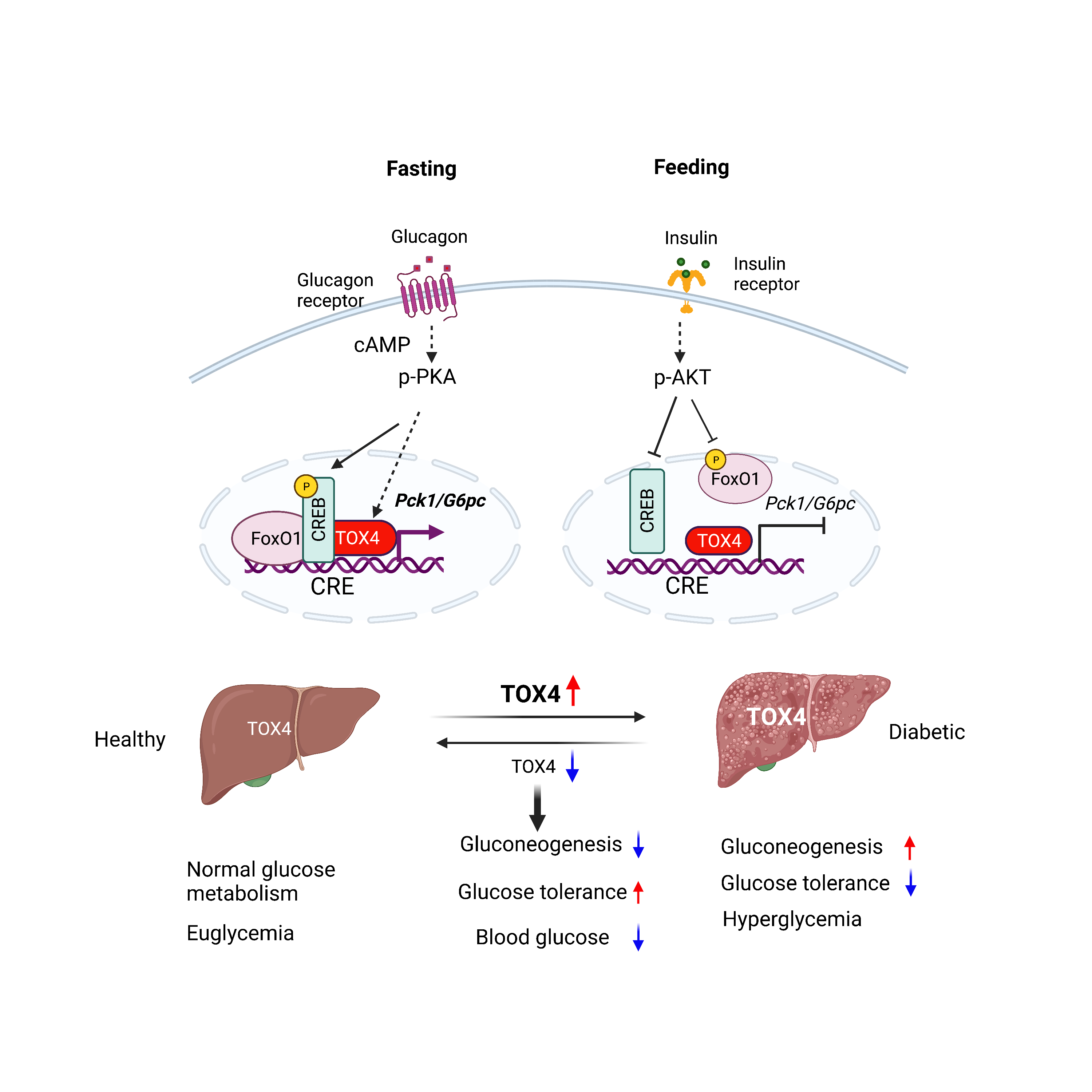
Project 1. Role of TOX4 in liver lipid metabolism and NAFLD
The liver plays a central role in systemic glucose and lipid homeostasis. Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease and is strongly associated with obesity (70-90%) and T2D (80%). NAFLD and T2D frequently coexist and synergistically contribute to cardiometabolic co-morbidities. T2D and NAFLD are driven by complex inherited and environmental factors related to obesity, insulin resistance, and dyslipidemia. Given this complexity, understanding T2D and NAFLD requires an integrated approach leveraging genetics, cell biology, metabolism, and animal experimentation. Our research training spans all these areas with a focus on liver metabolism. In the next few years, we plan to delve into the regulation of liver glucose and lipid metabolism through a hypothesis-driven project focused on TOX4 and a discovery-based project on organ interactions.
Project 2: Explore organ crosstalk in the progression of NAFLD/T2D
The liver is arguably the central organ regulating glucose and lipid homeostasis. However, the pathological changes of liver in T2D/NAFLD are inseparable from crosstalk with other organs such as adipose, gut, pancreas, heart, muscle and brain via endocrine, nutritional, and neuronal cues. Here we propose this exploratory project to identify research directions and translational opportunities.