Our primary research interests focus on the identification of novel mechanisms and therapies for cardiac failure in diabetes and metabolic syndrome. Particular emphasis is put on the elucidation of the genetic and cellular mechanisms underlying the pathophysiology of diabetic cardiomyopathy. By identifying genes and molecular signaling pathways that influence cardiac physiology and related cardiometabolism dysregulation, we aim to provide new insights in the genetic and cellular the mechanisms that regulate diabetic heart failure and risk of metabolic disease with an eye toward genetic and pharmacological therapeutic interventions.
Current Research Interests
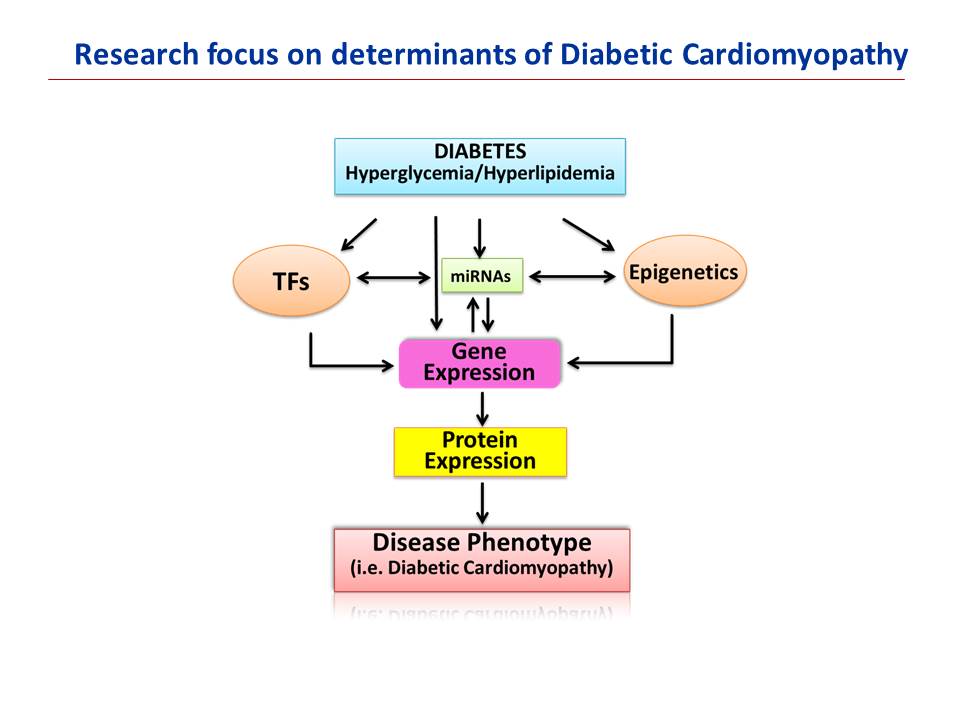
Defining the molecular, genetic and epigenetic determinants of diabetic cardiomyopathy: The focus in this area is to dissect and understand the genomic and epigenetic regulatory mechanisms responsible for cardiac dysfunction in diabetes and insulin resistant conditions. Diabetes and its cardiovascular complications are very complex. The genetic and cellular mechanisms underlying the pathophysiology of diabetic cardiomyopathy are not well understood. Our approach is to use genomics and proteomics tool to interrogate the stochastic movements in gene and protein expression profiles accompanying diabetic cardiomyopathy in human and animal models. Our objective is to identify, validate and physiologically characterize changes in molecules and pathways that spur the onset of diabetes-induced heart failure, alter disease severity, speed or slow disease progression, or have a protective effect. Through a number of currently active projects, we hope to unravel mechanisms and identify genes/proteins that could serve as determinants of diabetic cardiomyopathy, thereby defining novel targets for therapeutic intervention.
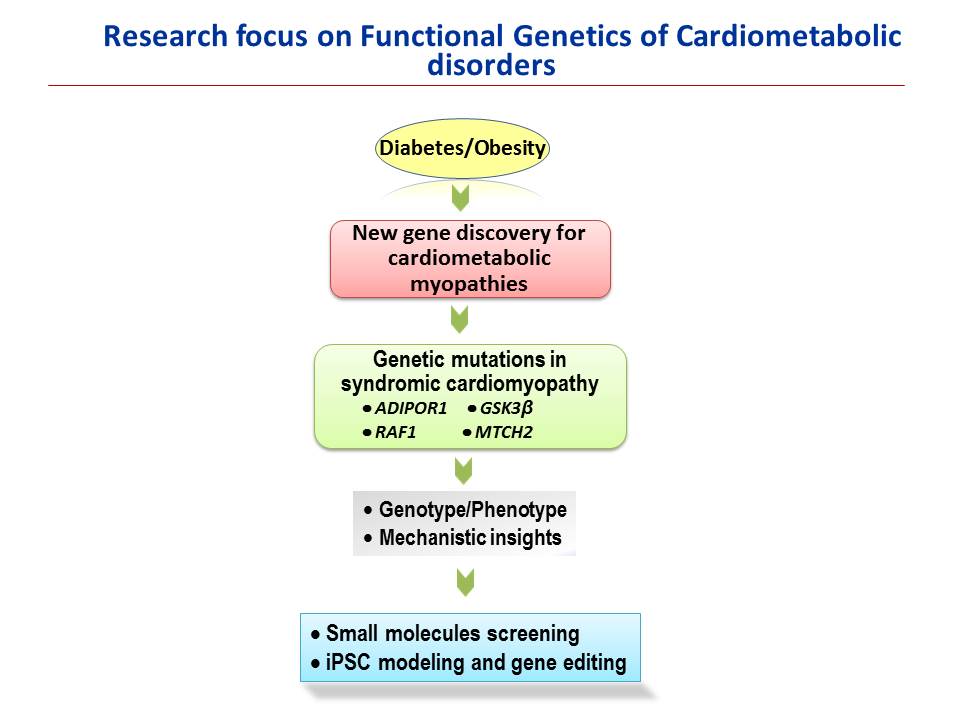
Functional-Molecular genetics and cardiometabolic disorders: Our group has also been interested in genetic linkage studies associating human mutation genes with cardiomyopathies and diabetes. The identification of genetic causes of the individual risk factors and the underlying genetic factors that unify their association in the metabolic syndrome are not known. Through our genetic linkage studies associating human mutations with hypertrophic cardiomyopathy and diabetes we aim, to provide new mechanistic insights and potential therapeutic targets for a novel concept of metabolic syndrome-cardiomyopathy axis in human cardiovascular disorders. Our efforts have thus far identified a number of novel functional mutations in hypertrophic cardiomyopathy-associated genes and diabetic genes. Work is underway to unravel the direct patho-functional impact of these human mutations on cardiac structure and function, molecular pathogenesis and signaling mechanisms. These studies will eventually establish a platform for futures studies that include high-throughput mutants-targeted drug development and gene correction by CRISPR- and AAV-mediated genome editing using patients’ specific iPSCs to treat cardiomyopathies.
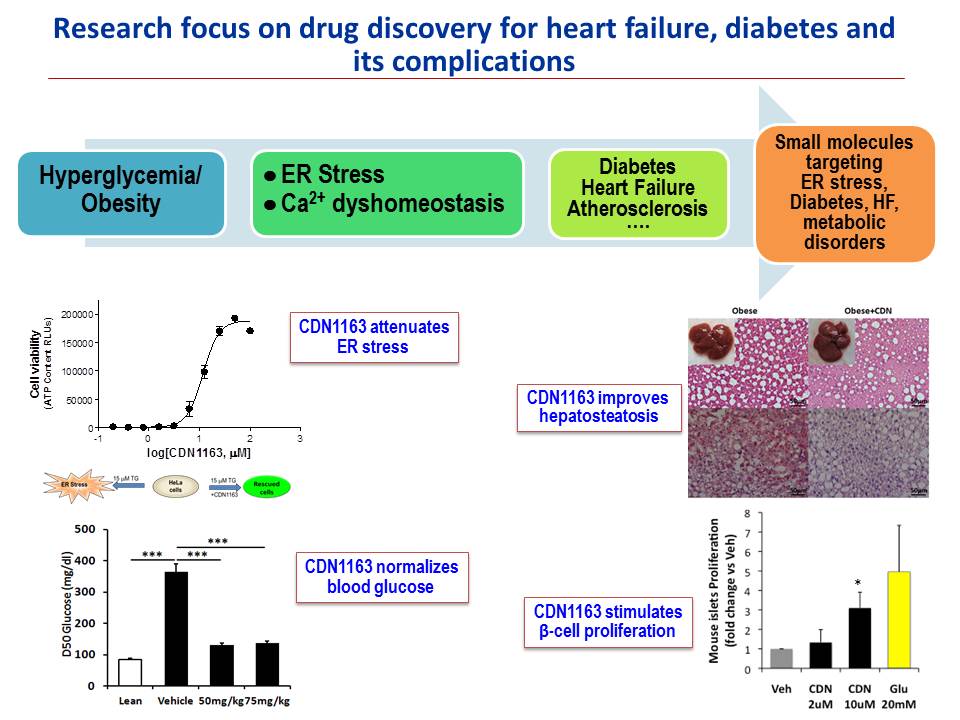
Investigational drug discovery for heart failure, diabetes and its complications: T2DM treatment has been revolutionized in the past decade, nevertheless significant unmet need remains for products that can offer better glycemic control as well as the prevention and cure of diabetic complications, such as diabetic cardiovascular disease. Our goal in this research area is to develop novel SERCA-based small molecules suitable for clinical development as novel therapeutic potential to treat heart failure, diabetes and other metabolic diseases. The importance of this project lies in the targeted biology (ER stress) and the mechanism of action (SERCA activation). Several active pipeline compounds have already been identified and work is underway to optimize and functionally validate existing and newly synthesized scaffolds using medicinal chemistry and analoging strategies, comparative modeling and target-based/ligand-based virtual screening. Multiple cheminformatics tools are used to support hit expansion and structure-activity relationship (SAR) studies around the validated hits to facilitate discovery of compounds suitable for further development as SERCA-based therapeutic modalities for diabetes and its complications, and heart failure.