Welcome to
DAI LAB
SYNAPTOPATHY OF NEUROPSYCHIATRIC DISORDERS
RESEARCH
THE ULTIMATE AIM OF OUR “SYNTHERAPY” LAB IS TO FIND SYNAPTIC THERAPY TO TREAT GENETIC FACTOR OR STRESS FACTOR INDUCED NEUROPSYCHIATRIC DISORDERS.
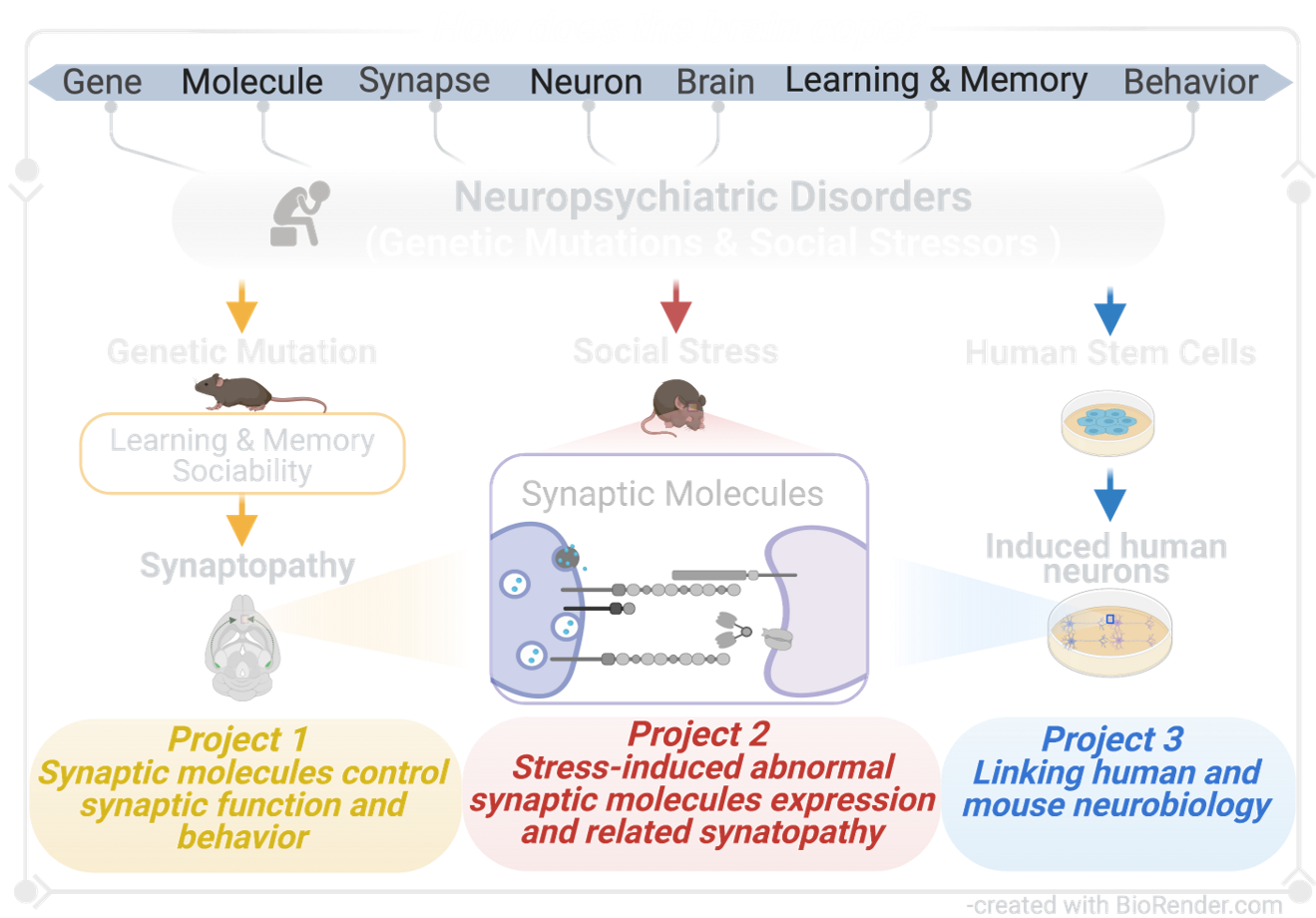
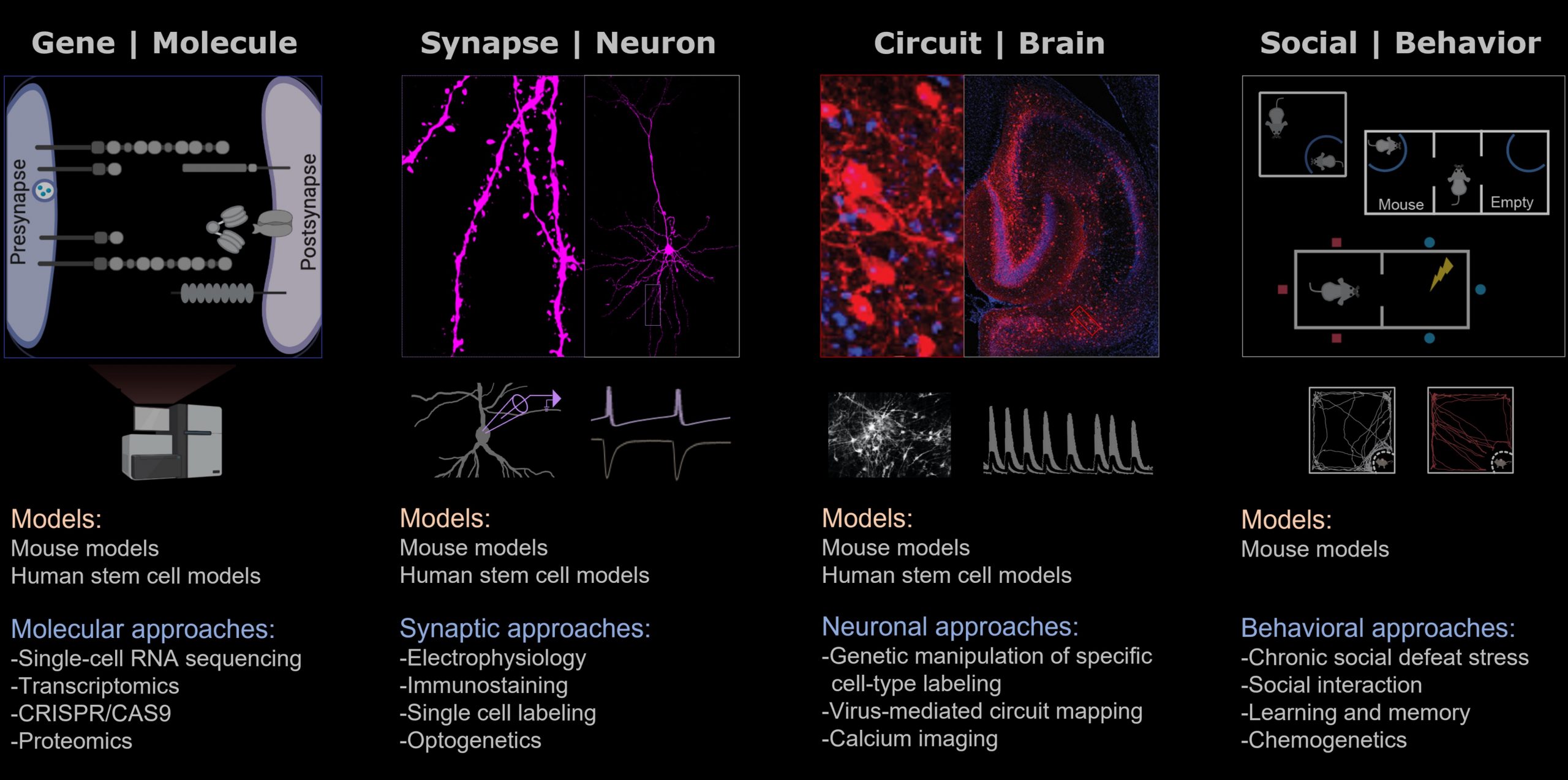
TO ACHIEVE THIS, we combine cutting-edge experimental techniques, including RNA sequencing, CRISPR/CAS9, Immunostaining, Electrophysiology, and Behaviroal test, using mouse models and human stem cell models to provide key insights into the interdependence between synapse function and behavior, and generate a comprehensive molecular understanding of both, the adaptive nature of healthy brain function as well as pathological forms of genetic mutation-induced and stress-induced neuropsychiatric disorders.
PEOPLE

Jinye Dai, PhD
Assistant Professor
Department of Pharmacological Sciences
Department of Neuroscience
Member of the Friedman Brain Institute
jinye.dai@mssm.edu
Dr. Dai received her PhD degree from the Institute of Biophysics, the Chinese Academy of Sciences where she studied the fundamental mechanisms of synaptic transmission with Prof. Jianyuan Sun. Then, Dr. Dai completed her postdoctoral study at Stanford University School of Medicine with Prof. Thomas Südhof. Dr. Dai’s research interest is directed at better understanding of basic molecular mechanisms of brain synaptic function and neuropsychiatric disorders.

Eric Purisic
Research Associate
eric.purisic@mssm.edu
I received my BS in Integrative Neuroscience with a concentration on Cell and Molecular Neuroscience from Fordham University in 2022. While an undergraduate, I began working in Dr. Marija Kundakovic’s lab where I studied the effects of early-life stress on the expression of a set of X-linked genes escaping X-inactivation. Due to this work, I have become very interested in the effects of stress on the brain on a cellular and molecular level, especially in specific brain regions involved in stress-related pathology. As a result of these interests, I joined the Dai Lab at the beginning of 2023 as a research associate. My long-term goal is to pursue a PhD in Neuroscience and advance our understanding of the cellular and molecular mechanisms of stress response and their role in the development of neuropsychiatric disorders. My interests outside of the lab include reading, playing video games, cooking, watching reality TV, and listening to 50,000 minutes of Spotify a year.

Joshua Stamos
Postdoctoral Fellow
joshua.stamos@mssm.edu
Dr. Stamos received his Ph.D. from Rutgers University where he worked with Dr. Mark West. There he studied the effects of binge eating behavior on cue procession in Nucleus Accumbens using single unit recordings. Following this he worked as a postdoc for the VA Hospital in New Jersey with Dr. Kevin Beck. Dr. Stamos’ current research interests involve understanding neurobiological mechanisms for how stress-induced behavioral changes affect neural connection on the synaptic level.

Tianhua Wang
Visiting Graduate Student
tianhua.wang@mssm.edu
I received my master’s degree from East China Normal University under the supervision of Dr. Huimin Wang and Dr. Chunxia Li. I am a PhD candidate at Philipps-University Marburg with Dr. Markus Wöhr. I am currently visiting Dr. Jinye Dai lab within a collaboration. My research focuses on the social affective communication in rodents and its serotonin implicated molecular mechanism. My research interest is to understand the molecular mechanisms of social behavior and the related psychiatric disorders like autism spectrum disorder. I have a very smart and sweet cat – Her name is Lucky and she does bring me luck.

Fiona Yeung
High School Student
Fiona is a current senior at John F. Kennedy High School, Bellmore. Since the summer of 2023, she has worked under the mentorship of Dr. Jinye Dai and Dr. Joshua Stamos, where she has been able to explore various experimental techniques to investigate neurodevelopmental disorders from stress-induced changes in brain regions. Looking ahead, Fiona hopes to continue pursuing her passion for research during college and learn more about neurobiology and cellular and molecular biology. Fiona enjoys baking sweets, playing the violin, and tending to her aquariums in her free time.
PUBLICATIONS
paper highlights, see also google scholar and pubmed
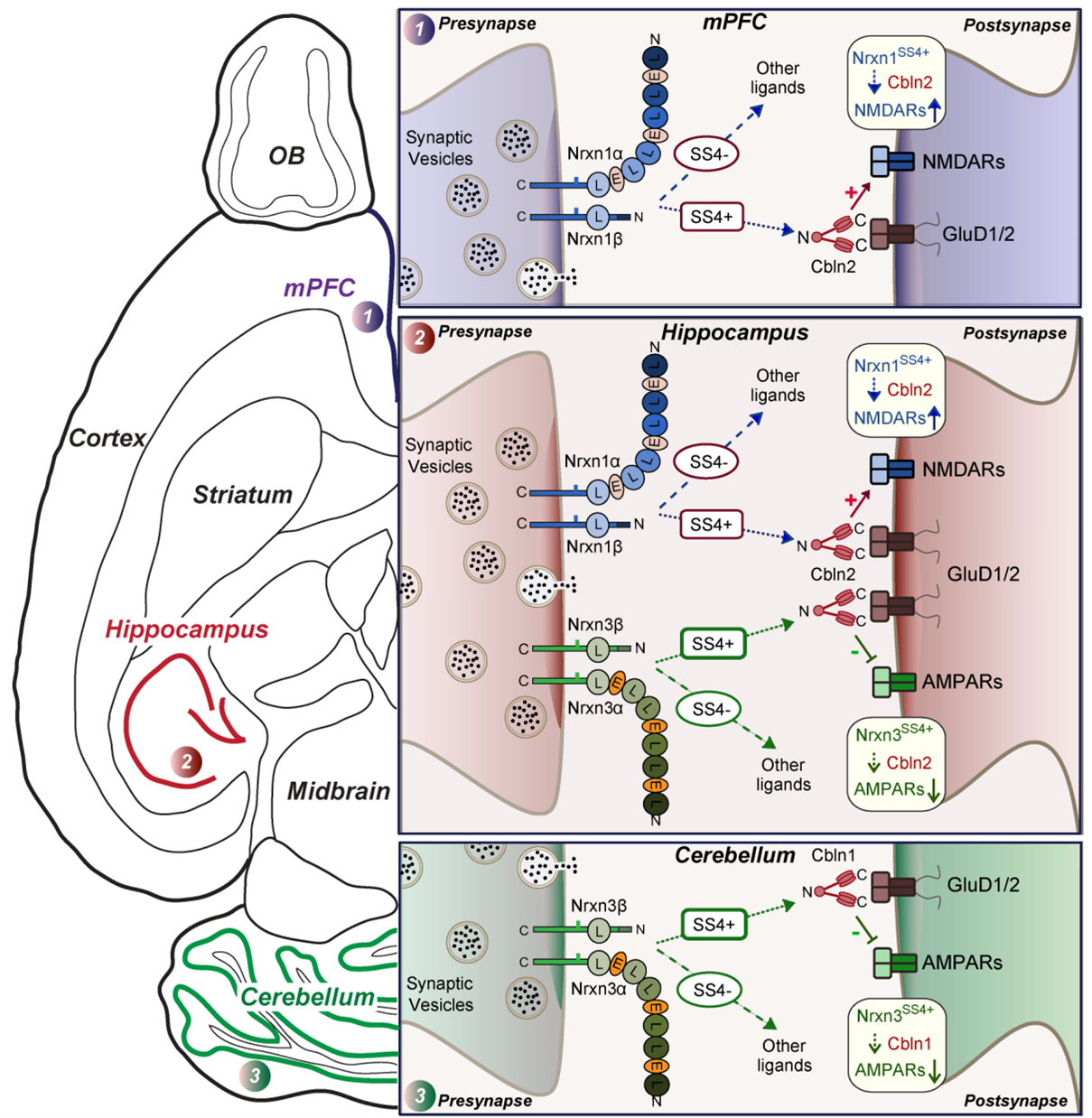
Dai J, Liakath-Ali K, Golf SR, Südhof TC. Distinct Neurexin-Cerebellin Complexes Control AMPA-and NMDA-Receptor Responses in a Circuit-Dependent Manner. elife, 2022 LINK
At CA1→subiculum synapses, alternatively spliced neurexin-1 (Nrxn1SS4+) and neurexin-3 (Nrxn3SS4+) enhance NMDA-receptors and suppress AMPA-receptors, respectively, without affecting synapse formation. Nrxn1SS4+ and Nrxn3SS4+ act by binding to secreted cerebellin-2 (Cbln2) that in turn activates postsynaptic GluD1 receptors. Whether neurexin-Cbln2-GluD1 signaling has additional functions besides regulating NMDA- and AMPA-receptors, and whether such signaling performs similar roles at other synapses, however, remains unknown. Here, we demonstrate using constitutive Cbln2 deletions in mice that at CA1→subiculum synapses, Cbln2 performs no additional developmental roles besides regulating AMPA- and NMDA-receptors. Moreover, low-level expression of functionally redundant Cbln1 did not compensate for a possible synapse-formation function of Cbln2 at CA1→subiculum synapses. In exploring the generality of these findings, we examined the prefrontal cortex where Cbln2 was recently implicated in spinogenesis, and the cerebellum where Cbln1 is known to regulate parallel-fiber synapses. In the prefrontal cortex, Nrxn1SS4+-Cbln2 signaling selectively controlled NMDA-receptors without affecting spine or synapse numbers, whereas Nrxn3SS4+-Cbln2 signaling had no apparent role. In the cerebellum, conversely, Nrxn3SS4+-Cbln1 signaling regulated AMPA-receptors, whereas now Nrxn1SS4+-Cbln1 signaling had no manifest effect. Thus, Nrxn1SS4+- and Nrxn3SS4+-Cbln1/2 signaling complexes differentially control NMDA- and AMPA-receptors in different synapses in diverse neural circuits without regulating synapse or spine formation.
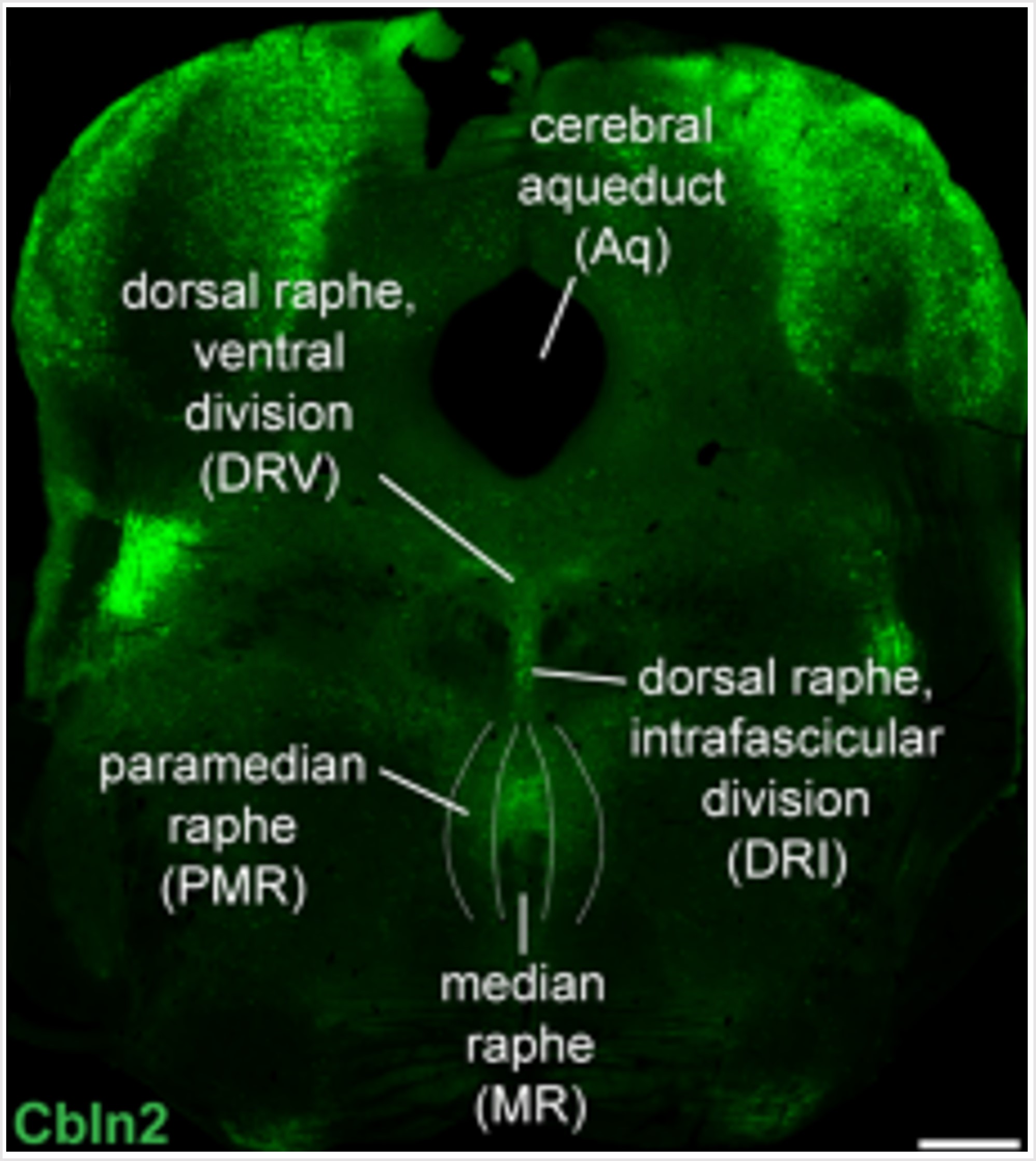
Seigneur E, Wang J, Dai J, Polepalli J, Südhof TC. Cerebellin-2 regulates a serotonergic dorsal raphe circuit that controls compulsive behaviors. Molecular Psychiatry, 2022 Jun; 26(12), 7509–7521. LINK
Cerebellin-1 (Cbln1) and cerebellin-2 (Cbln2) are secreted glycoproteins that are expressed in distinct subsets of neurons throughout the brain. Cbln1 and Cbln2 simultaneously bind to presynaptic neurexins and postsynaptic GluD1 and GluD2, thereby forming trans-synaptic adhesion complexes. Genetic associations link cerebellins, neurexins and GluD’s to neuropsychiatric disorders involving compulsive behaviors, such as Tourette syndrome, attention-deficit hyperactivity disorder (ADHD), and obsessive-compulsive disorder (OCD). Extensive evidence implicates dysfunction of serotonergic signaling in these neuropsychiatric disorders. Here, we report that constitutive Cbln2 KO mice, but not Cbln1 KO mice, display robust compulsive behaviors, including stereotypic pattern running, marble burying, explosive jumping, and excessive nest building, and exhibit decreased brain serotonin levels. Strikingly, treatment of Cbln2 KO mice with the serotonin precursor 5-hydroxytryptophan or the serotonin reuptake-inhibitor fluoxetine alleviated compulsive behaviors. Conditional deletion of Cbln2 both from dorsal raphe neurons and from presynaptic neurons synapsing onto dorsal raphe neurons reproduced the compulsive behaviors of Cbln2 KO mice. Finally, injection of recombinant Cbln2 protein into the dorsal raphe of Cbln2 KO mice largely reversed their compulsive behaviors. Taken together, our results show that Cbln2 controls compulsive behaviors by regulating serotonergic circuits in the dorsal raphe.
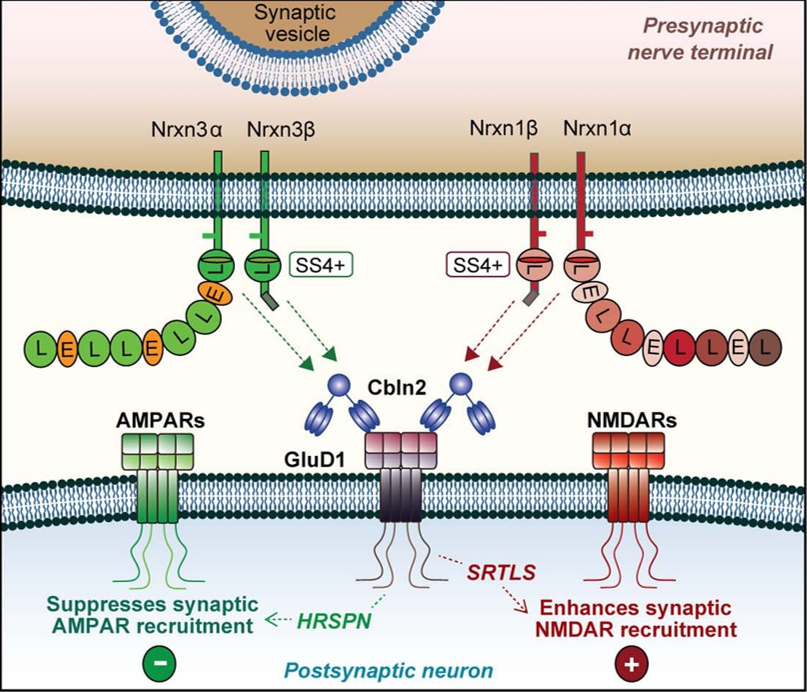
Ionotropic glutamate delta receptors 1 (GluD1) and 2 (GluD2) exhibit the molecular architecture of postsynaptic ionotropic glutamate receptors, but assemble into trans-synaptic adhesion complexes by binding to secreted cerebellins that in turn interact with presynaptic neurexins. It is unclear whether neurexin–cerebellin–GluD1/2 assemblies serve an adhesive synapse-formation function or mediate trans-synaptic signalling. Here we show in hippocampal synapses, that binding of presynaptic neurexin–cerebellin complexes to postsynaptic GluD1 controls glutamate receptor activity without affecting synapse numbers. Specifically, neurexin-1–cerebellin-2 and neurexin-3–cerebellin-2 complexes differentially regulate NMDA (N-methyl-D-aspartate) receptors and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors by activating distinct postsynaptic GluD1 effector signals. Of note, minimal GluD1 and GluD2 constructs containing only their N-terminal cerebellin-binding and C-terminal cytoplasmic domains, joined by an unrelated transmembrane region, fully control the levels of NMDA and AMPA receptors. The distinct signalling specificity of presynaptic neurexin-1 and neurexin-3 is encoded by their alternatively spliced splice site 4 sequences, whereas the regulatory functions of postsynaptic GluD1 are mediated by conserved cytoplasmic sequence motifs spanning 5–13 residues. Thus, GluDs are signalling molecules that regulate NMDA and AMPA receptors by an unexpected transduction mechanism that bypasses their ionotropic receptor architecture and directly converts extracellular neurexin–cerebellin signals into postsynaptic receptor responses.
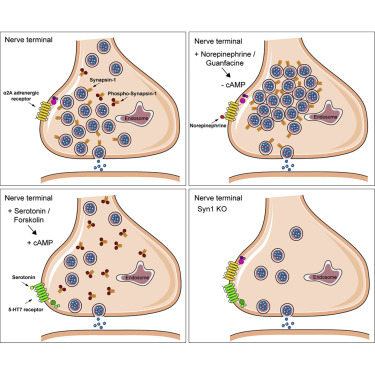
Patzke C, Brockmann MM, Dai J, Gan KJ, Grauel MK, Fenske P, Liu Y, Acuna C, Rosenmund C, Südhof TC. Neuromodulator Signaling Bidirectionally Controls Vesicle Numbers in Human Synapses. Cell, 2019 Oct; 179 (2), 498-513. LINK
Neuromodulators bind to pre- and postsynaptic G protein-coupled receptors (GPCRs), are able to quickly change intracellular cyclic AMP (cAMP) and Ca2+ levels, and are thought to play important roles in neuropsychiatric and neurodegenerative diseases. Here, we discovered in human neurons an unanticipated presynaptic mechanism that acutely changes synaptic ultrastructure and regulates synaptic communication. Activation of neuromodulator receptors bidirectionally controlled synaptic vesicle numbers within nerve terminals. This control correlated with changes in the levels of cAMP-dependent protein kinase A-mediated phosphorylation of synapsin-1. Using a conditional deletion approach, we reveal that the neuromodulator-induced control of synaptic vesicle numbers was largely dependent on synapsin-1. We propose a mechanism whereby non-phosphorylated synapsin-1 “latches” synaptic vesicles to presynaptic clusters at the active zone. cAMP-dependent phosphorylation of synapsin-1 then removes the vesicles. cAMP-independent dephosphorylation of synapsin-1 in turn recruits vesicles. Synapsin-1 thereby bidirectionally regulates synaptic vesicle numbers and modifies presynaptic neurotransmitter release as an effector of neuromodulator signaling in human neurons.
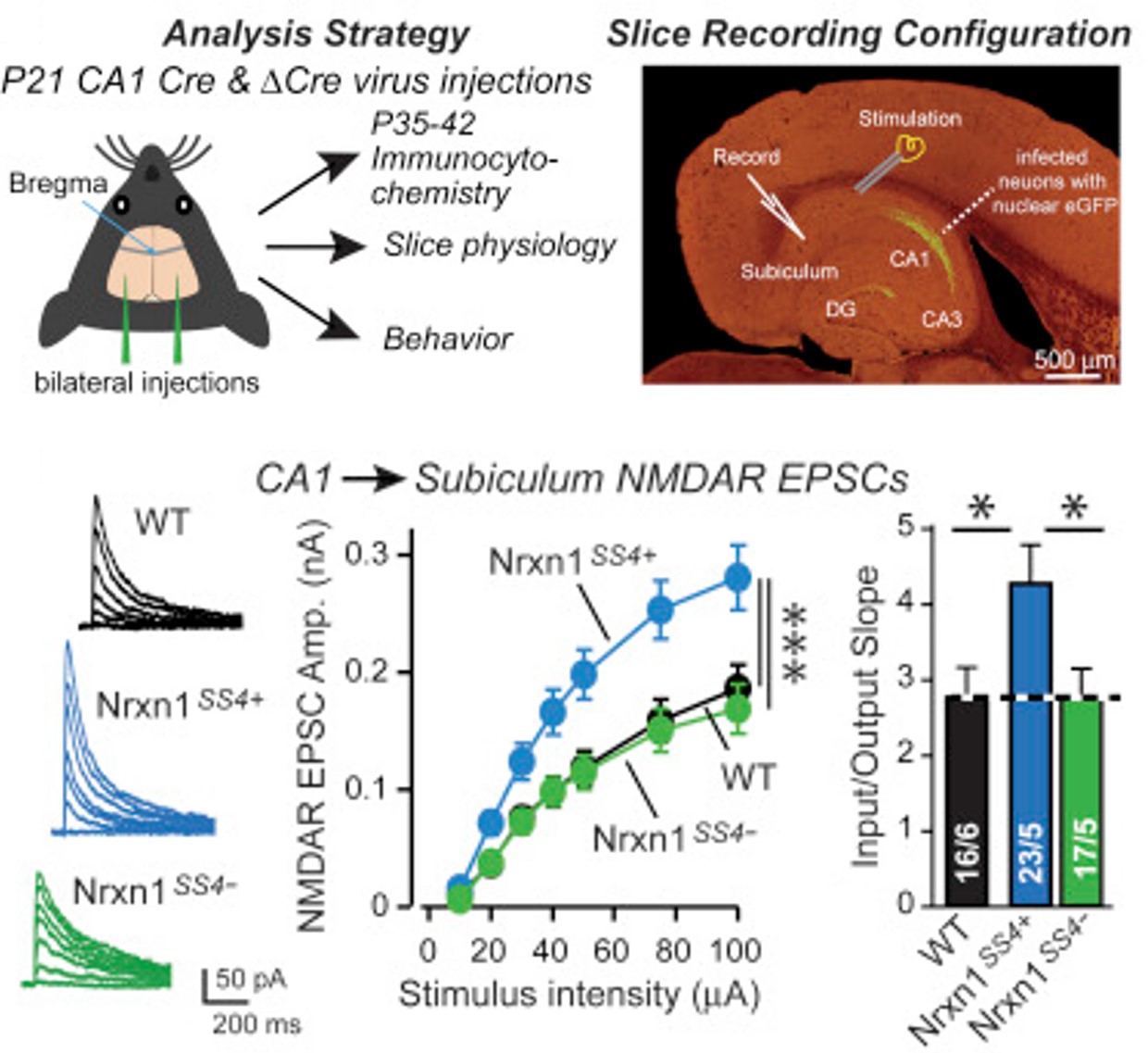
Dai J, Aoto J, Südhof TC. Alternative splicing of presynaptic neurexins differentially controls postsynaptic NMDA and AMPA receptor responses. Neuron, 2019 Jun; 102 (5), 993-1008. LINK
AMPA- and NMDA-type glutamate receptors mediate distinct postsynaptic signals that differ characteristically among synapses. How postsynaptic AMPA- and NMDA-receptor levels are regulated, however, remains unclear. Using newly generated conditional knockin mice that enable genetic control of neurexin alternative splicing, we show that in hippocampal synapses, alternative splicing of presynaptic neurexin-1 at splice site 4 (SS4) dramatically enhanced postsynaptic NMDA-receptor-mediated, but not AMPA-receptor-mediated, synaptic responses without altering synapse density. In contrast, alternative splicing of neurexin-3 at SS4 suppressed AMPA-receptor-mediated, but not NMDA-receptor-mediated, synaptic responses, while alternative splicing of neurexin-2 at SS4 had no effect on NMDA- or AMPA-receptor-mediated responses. Presynaptic overexpression of the neurexin-1β and neurexin-3β SS4+ splice variants, but not of their SS4− splice variants, replicated the respective SS4+ knockin phenotypes. Thus, different neurexins perform distinct nonoverlapping functions at hippocampal synapses that are independently regulated by alternative splicing. These functions transsynaptically control NMDA and AMPA receptors, thereby mediating presynaptic control of postsynaptic responses.
CURRENT OPENINGS
Postdoctoral Fellow
We seek a highly motivated scientist with strong organizational and communication skills to join our enthusiastic, collaborative, and creative team in an outstanding scientific environment. Please be sure to send your CV, research statement (a description of research experience and scientific interests), career goals, and the names and contact information of 3 references to Dr. Jinye Dai at jinye.dai@mssm.edu. LINK for DETAILS
Research Assistant
We also seek a highly motivated research assistant with strong organizational and communication skills to join our team. Please be sure to send your CV, research experience, career goals, and the names and contact information of 2 references to Dr. Jinye Dai at jinye.dai@mssm.edu. LINK for DETAILS
Graduate Student & M.D./Ph.D. Students
Interested prospective graduate students and M.D./Ph.D. students should apply to the Graduate School at Mount Sinai. Students who are interested in rotation projects, please contact Dr. Jinye Dai at jinye.dai@mssm.edu for more information.

