Projects
1. Decoding itch signaling mechanisms in the skin
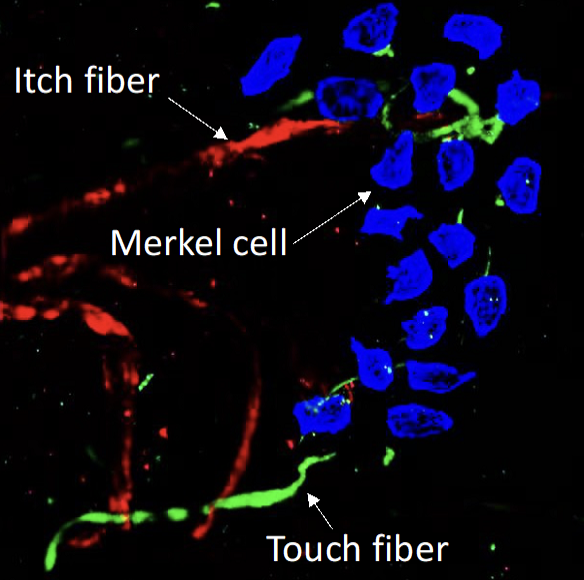
Both spontaneous and mechanical itch responses originate in the skin. Previous research has identified the Merkel cell-slowly adaptive Ab touch fiber complex as a key regulator of mechanical itch in normal conditions. However, in the context of aging or chronic itch associated with dry skin, the loss of Merkel cells removes the inhibition on mechanical itch, leading to the development of alloknesis (mechanical itch sensitization). Our studies have also shown that surviving Merkel cells in mice, following experimental dry skin treatment, can establish a functional connection with MrgprA3-expressing itch fibers and promote spontaneous itch. Notably, the mechanosensitive Piezo2 channels expressed by Merkel cells are essential for inhibiting mechanical itch and promoting spontaneous itch through the activation of slowly adaptive Ab fibers and C-pruriceptors, respectively. These findings have significantly contributed to our understanding of how Piezo2 channels and Merkel cells influence itch signaling in the skin. However, the mechanisms by which Merkel cells respond to tissue injury, skin inflammation, and aging, as well as the impact of these changes in Merkel cell biology on their interaction with touch and itch fibers in the skin, remain incompletely understood. Furthermore, we will investigate whether Merkel cells play a role in the development of skin inflammation, in addition to their involvement in itch signaling.
2. Mechanisms of Interoception
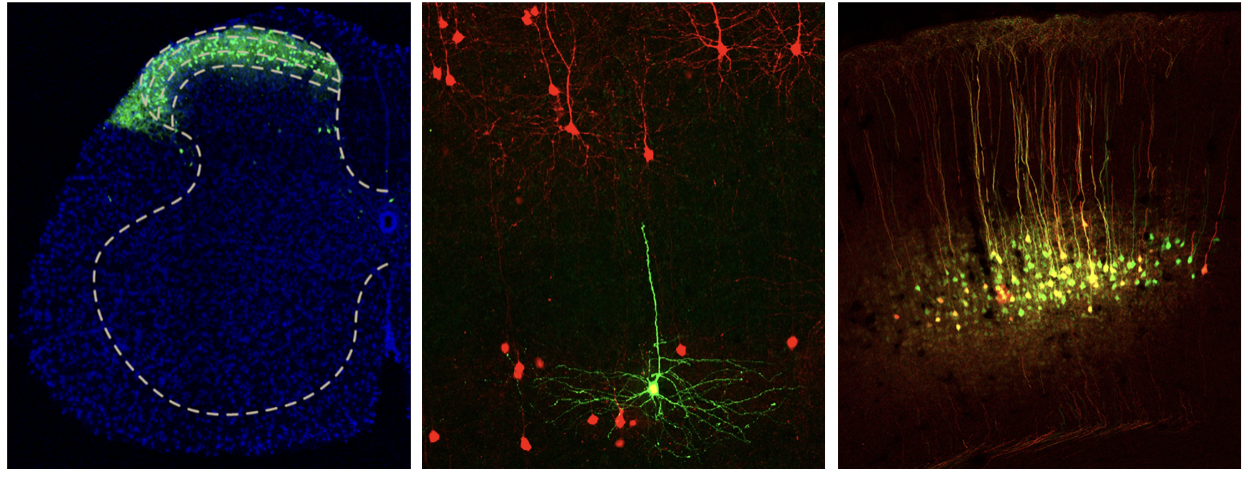
Sensory perceptions are initiated by a combination of external and internal cues, which play a crucial role in meeting our survival and psychological needs. The awareness of external stimuli, such as touch, sight, smell, sound, and taste, is known as exteroception. On the other hand, interoception refers to the perception of internal bodily states, including both unconscious processes like respiration and bowel movements, as well as conscious experiences like chest pain during a heart attack and intestinal cramps. Interoceptive signals are transmitted to the brain through two main pathways: the ascending viscerosensory pathway via the spinal cord, and the vagus sensory pathway through the nucleus tractus solitarii in the brainstem. However, our understanding of the CNS circuits responsible for processing interoceptive inputs from visceral organs is still limited. To address these gaps, we will utilize conventional genetic techniques and virally mediated intersectional genetics to identify neural circuits that are essential for both the sensory discriminative and affective components of visceral pain.
3. Affective dimension of pain and pain-associated coping behaviors
There are distinct coping behaviors between somatic and visceral nociceptive pathways. Pain originating from the colon is inescapable, leading to passive emotional coping. Conversely, cutaneous pain, such as the pain felt when grasping a hot plate, elicits both reflexive withdrawal behaviors and active emotional coping in the form of “fight or flight” responses. Our research aims to understand the encoding of the affective-motivational aspects of pain and self-caring behaviors in the spinal cord and brainstem. Additionally, we are investigating the impact of an inability to cope with pain on mental well-being. By gaining insights into these mechanisms, we can develop effective treatments for individuals who suffer from anxiety and depression associated with chronic pain
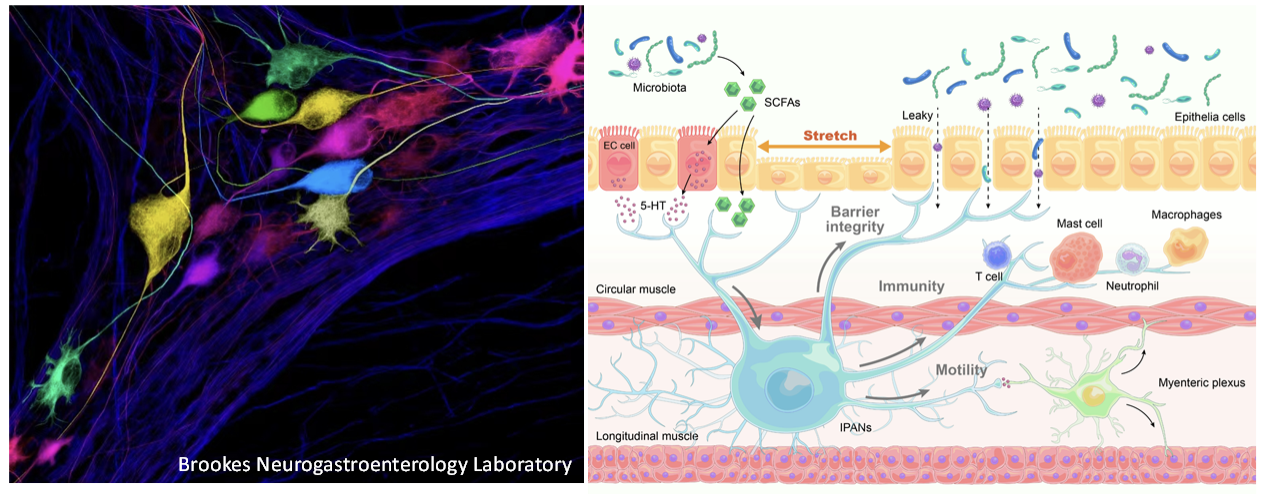
4. Function of the enteric nervous system
The gut serves as the primary site for digestion, absorption of nutrients, and waste excretion. Additionally, it houses a vast number of microorganisms, collectively known as the gut microbiota, which play a crucial role in influencing communication between the gut and the brain. This communication is essential for both immune regulation and brain health. Therefore, maintaining normal gut function is vital for overall health and well-being. Unlike other abdominal organs, the gastrointestinal (GI) tract possesses its own fully contained nervous system within its walls, known as the enteric nervous system (ENS) or the “second brain” in the gut. The ENS consists of various types of neurons, including sensory, interneurons, motor neurons, and glial cells. Together, these components detect the contents of the gut, regulate secretory function and intestinal movement, maintain immune balance, and preserve the integrity of the intestinal barrier. Although enteric neural circuits represent a significant therapeutic target for various GI disorders, progress in probing and controlling the diverse circuit elements of the ENS has been hindered by technological limitations. We have recently overcome technical obstacles and developed unique resources that allow us to access genetically defined and molecularly distinct neuronal populations in the ENS. By utilizing these innovative neurogenetic approaches, we aim to investigate the specific neuronal populations and neural circuits within the ENS and their impact on GI motility, immunity, and the integrity of the intestinal barrier in both normal and pathological conditions.