Our Research
Our research focuses on understanding the initiation of sensory experiences, such as pain and itch, at the barrier surfaces of the skin and visceral organs. Additionally, we investigate how these signals, both from external and internal sources, converge in the spinal cord and are processed by specific neural circuits. Furthermore, we are interested in understanding how animals display distinct coping behaviors in response to somatic and visceral pain, how external and internal sensations are integrated to influence emotional states underlying behavior, and how dysregulated interoception can lead to psychopathologies.
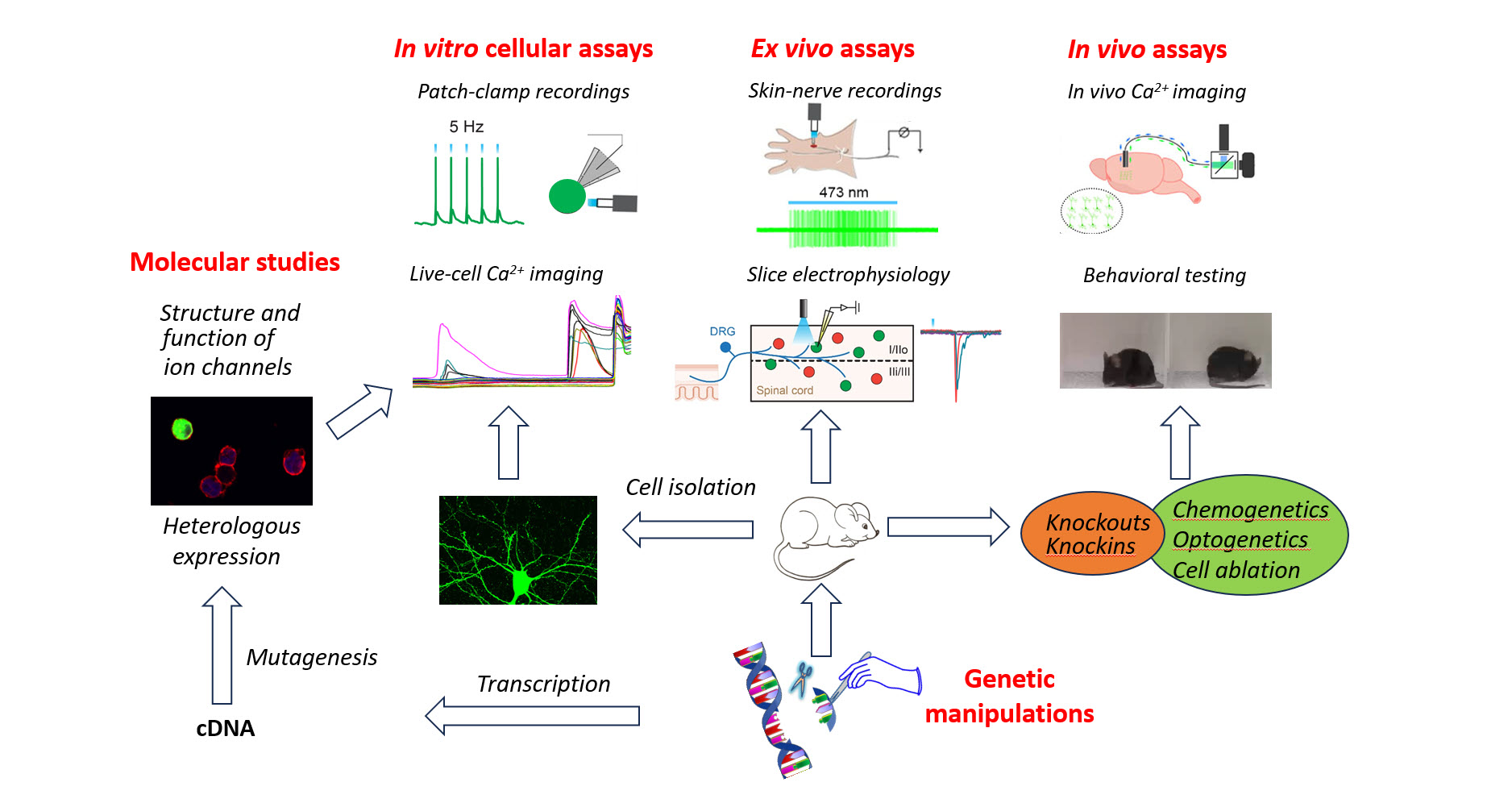
Our Approach
We employ multidisciplinary approaches, such as pharmacological, optogenetic, and chemogenetic manipulations, to study genetically defined and molecularly distinct neuronal populations. Additionally, we utilize a combination of neural tracing, in vivo imaging, electrophysiological recordings, molecular biology, and behavioral testing to gain insights into the molecular, cellular, and circuit mechanisms that underlie sensory processing in mice.