 CARDIAC REPAIR, STEM AND PROGENITOR CELL BIOLOGY
CARDIAC REPAIR, STEM AND PROGENITOR CELL BIOLOGY
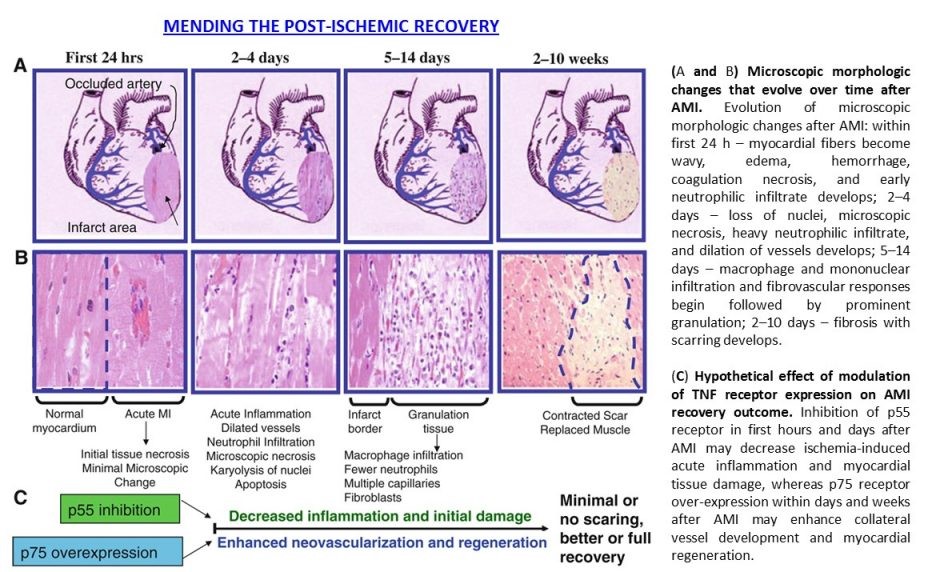
The focus of Dr. Goukassian’s research efforts has been on identifying the role of TNF in ischemia-induced inflammation, tissue regeneration, therapeutic angiogenesis and gene therapy. The research is aimed at development of treatments based on TNF-TNFR1/p55 and -TNFR2/p75 ligand-receptor interaction, specifically, (1) development of therapies to improve post-ischemic repair, regeneration and neovascularization processes; (2) development of universal platform therapeutics to improve stem cell and gene therapy outcomes in damaged tissues. TNF, a pro-inflammatory cytokine, is highly expressed in any damaged tissue, and plays a key role in the repair and regeneration processes via its two receptors, TNFR1 (p55) and TNFR2 (p75), whereby p55 mediates cytotoxic effects while p75 is pro-survival. Inflammation is an important phase of tissue regeneration processes. Perturbations in the spatial distribution of inflammatory cells, changes in the type and magnitude of the inflammatory infiltrate, disrupted temporal sequence, results in a persistent rather than resolved inflammatory phase and functional impairment of the damaged tissue. As a pro-inflammatory cytokine, TNF induces apoptosis and blocks stem and progenitor cell differentiation. The key finding of our research in this field with major therapeutic implications is that – restoration or overexpression of TNFR2/p75 (gene therapy) or inhibition of TNFR2/p55 (neutralizing antibody or small molecules) improves recovery in damaged tissue through inhibition of prolonged inflammation and cell death, promotion of therapeutic angiogenesis and development of new vascular network, thereby creating tissue environment for recruitment, incorporation and function of endogenous and exogenous stem cells and increasing survival of the resident differentiated cells and adult stem cells. This approach will improve stem and progenitor cell-therapy effectiveness and outcomes and could serve as a universal platform for stem cell and gene therapy.
CARDIOVASCULAR SPACE AND TERRESTRIAL RADIOBIOLOGY
NASA Human Research Program (HRP) identified space radiation as one of the space flight risk factors to the cardiovascular system. In addition, long-duration space missions such as work on ISS, future lunar, near Earth asteroid, and Mars missions, as well as emerging civilian space travel activities, necessitate ground-based studies on degenerative cardiovascular risk assessment of space radiation effect. His current NASA-funded research work is focused in the area of Space Radiobiology, specifically, evaluation of space radiation-induced damage to cardiomyocytes and cardiac ECs and evaluation of space radiation-associated long-term degenerative risks to cardiovascular system. Dr. Goukassian’s earlier research evolved from formal training in DNA damage and repair, genetics and immunology, to a recent focus on using integrated physiological approaches along with genomic, proteomic and epigenetic methodologies to understand molecular mechanisms of space radiation-induced long-term cardiovascular degenerative risks, development of mouse models for evaluation of space radiation-induced Excess Relative Risks-ERR, development of predictive biomarkers for estimation of ERR, development of countermeasures for NASA’s future long-duration space missions to Moon, near Earth asteroids and Mars. Researched cardiovascular disease risks for astronauts from low dose radiation exposures. Assisted NASA in developing heart disease risk estimates and proposed measures to prevent Earth-bound population from radiation-induced cardiovascular diseases in cancer patients receiving “standard of care” conventional and particle radiotherapy. We found, that low dose exposures of charged particles present in the space IR environment may have significant impact on CV and bone marrow system. These studies have significant implications for NASA’s efforts to develop heart disease risk estimates for astronauts, as well as for prevention of radiation-induced cardiovascular diseases in cancer patients receiving conventional and particle radiotherapy.
 DEVELOPMENT OF NOVEL ANTICANCER THERAPEUTICS
DEVELOPMENT OF NOVEL ANTICANCER THERAPEUTICS
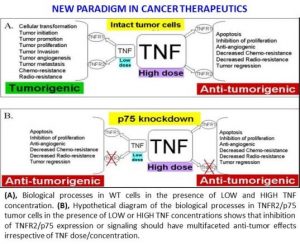
Since 2011, Dr. Goukassian has been working on the development of novel anti-cancer treatment modalities based on inhibition of TNF signaling via selective blocking of one of its receptors (TNFR2 p75) for treatment of various human tumors including, but not limited to, lung, breast and melanoma. As result of this work we developed new monoclonal antibodies that block the pro-survival receptor of TNF. Our studies showed that when pro-survival TNF2 is blocked in the host (p75KO mice) or cancer cells themselves (knockdown TNFR2 in cancers cells with p75 shRNA) there is very significant inhibition of tumor angiogenesis and more than 50-60% reduction in tumor growth. What we also found is that an initial inhibition of TNFR2 induced the production and release of Tumor Necrosis Factor (death of cancer cells) into tumor tissue. Therefore, the TNF naturally released by dying tumor cells produce their “own poison” as long as TNFR2 is neutralized in the cells of tumor tissue. This approach offers paradigm changing cancer treatment targeting proliferative and silent cancer cells, cancer associated tumor stroma and endothelial cells providing an effective method of “priming the tumor for self-destruction”.
