CD16a/b antibody
CD16a is the human Fc gamma receptor that triggers antibody-dependent cellular cytotoxicity by NK cells and functions coordinately with two other activating and one inhibitory receptors to trigger antibody-dependent cellular phagocytosis by macrophages, but CD16a undergoes proteolytic shedding by ADAM-17. The shedding downregulates CD16a surface expression. Our laboratory developed the novel mAb F9H4 that binds CD16a and inhibits the shedding. F9H4 retains CD16a on the surface of NK and myeloid cells, and it binds CD16b, which is expressed by neutrophils, and inhibits its shedding. F9H4-mediated inhibition of CD16a shedding promotes NK cell effector functions against anti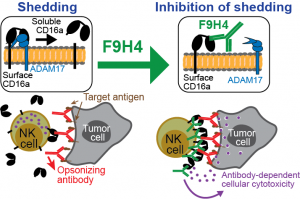 body-opsonized tumor cells and inhibits tumor growth. This study, published in Nature Communications (2025 Nov 11;16(1):9915), is the first to demonstrate that a CD16a/b-targeted antibody inhibits the shedding in a substrate-specific manner to promote anti-tumor innate immunity.
body-opsonized tumor cells and inhibits tumor growth. This study, published in Nature Communications (2025 Nov 11;16(1):9915), is the first to demonstrate that a CD16a/b-targeted antibody inhibits the shedding in a substrate-specific manner to promote anti-tumor innate immunity.
MICA/B antibody
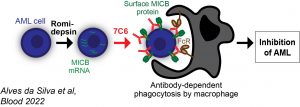
Malignant transformation induces expression of danger signals, such as MICA and MICB (abbreviated as “MICA/B”). Our lab uses MICA/B as leukemia antigens, by applying a monoclonal antibody against them to acute myeloid leukemia (AML) models. The antibody is called “7C6” and it inhibits the shedding of MICA/B by various cancer cells (Science 2018, 359(6383):1537). We recently discovered that 7C6 potently inhibits the outgrowth of AML in immunocompetent mice through antibody-dependent phagocytosis by macrophages against leukemia cells. Our recent study published in Blood (2022, 139(2):205) also revealed that romidepsin, a histone deacetylase inhibitor, synergizes with 7C6 to induce ultra-high levels of MICA/B in human leukemia cells and such effect enables phagocytosis by macrophages.
Development of new antibodies against other targets
Proteolytic cleavage is a post-translational modification frequently applied to a broad spectrum of surface proteins, such as cytokine receptors, cell adhesion molecules, growth factors, etc. Although proteolytic cleavage can be inhibited with small molecules that target proteases, these enzymes have broad specificity and are expressed by multiple cell types. Such small molecule inhibitors cause unspecific blockade of proteolytic cleavage and pleiotropic effects if administered in vivo. In contrast, our idea is to inhibit proteolytic cleavage in a substrate specific manner to enable physiologically relevant studies. Antibody-mediated inhibition of proteolytic cleavage represents a new research niche from our lab. We are currently developing additional antibodies that inhibit cleavage of a diverse panel of surface proteins.
