Project 1: Double-Trouble and DNA Damage Repair (DDR) Deficiency in Cancer
The normal structure of DNA is essential for healthy cell growth; however, DNA can sometimes become damaged or mutated. Pathological variants in DNA damage repair (DDR) pathway genes are prevalent in nearly every type of cancer, including a significant subset of men who develop metastatic castration-resistant prostate cancer (mCRPC), the most lethal form of the disease. Furthermore, the frequency of pathological DDR alterations is significantly higher in men with high-risk localized prostate cancer compared to those with low risk localized disease.
This observation has far-reaching clinical implications. For instance, aberrations in DDR pathway components could help identify individuals at the highest risk of developing mCRPC, and treatment strategies could be tailored based on somatic or germline findings. Our research revealed that in nearly 50% of tumors from men with mCRPC, the key DDR gene BRCA2 is deleted or non-functional, along with its chromosomal neighbor, RB1 (located at 13q). We demonstrated that the doubling down of both BRCA2 and RB1 is associated with disease progression, ultimately leading to fatal mCRPC. Our goal is to deepen our understanding of prostate cancer biology and to investigate how this DOUBLE TROUBLE, and modifications in other DDR genes, contribute to the aggressiveness of prostate cancer and its likelihood of evolving into mCRPC.
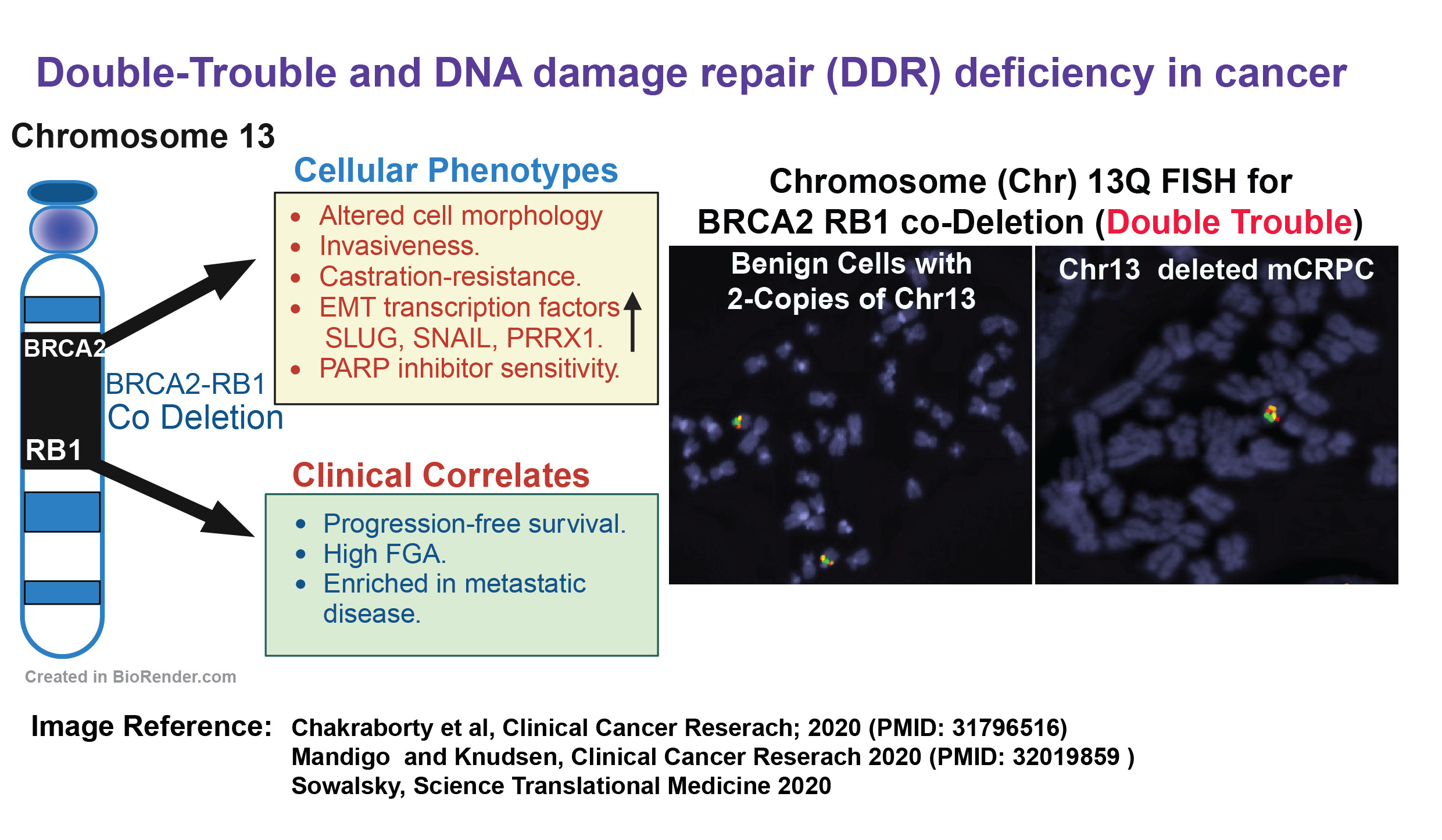
Project 2: Mechanisms of PARP Inhibitor Resistance and How to Overcome It
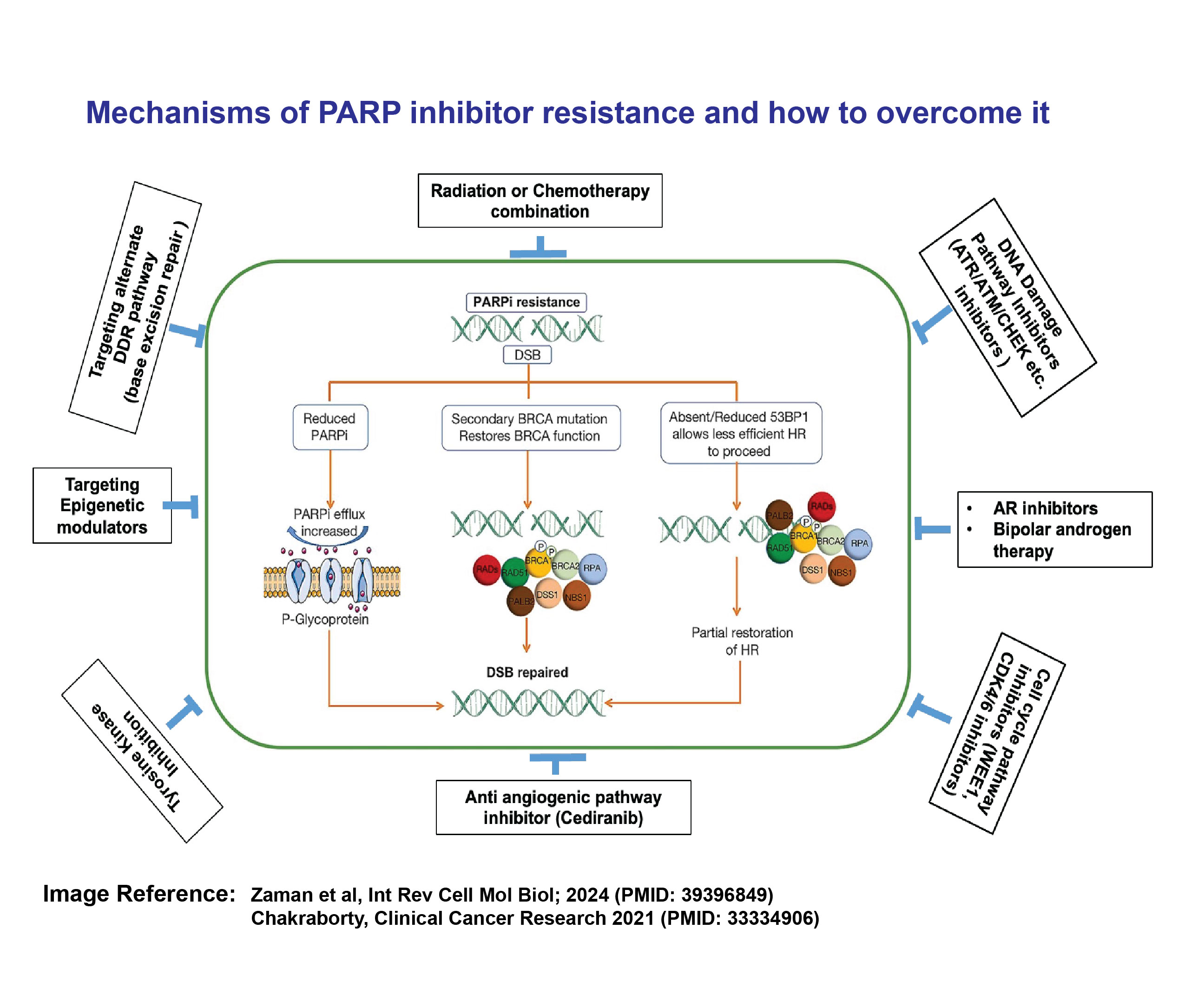
Cancer cells with DNA damage repair (DDR) alterations are particularly sensitive to drugs that inhibit a protein known as poly (ADP-Ribose) polymerase (PARP). Landmark clinical trials, including TRITON2, TOPARP-B, TALAPRO-2, and PROfound, have demonstrated that PARP inhibitors (PARPi), such as olaparib, rucaparib, and talazoparib, significantly improve radiological progression-free survival (rPFS) in metastatic castration-resistant prostate cancer (mCRPC) patients who harbor germline and somatic mutations in BRCA2 and 13 other homologous recombination repair (HRR) genes.
These inhibitors work by trapping PARP, which impairs its activity and increases DNA damage, ultimately leading to cell death through a process known as synthetic lethality. However, the therapeutic response to these inhibitors lasts only 3 to 4 months, after which the cancer often becomes resistant to PARP inhibitors. Cancer cells can develop resistance through various mechanisms, including secondary reversion mutations in DNA repair pathway genes, enhanced drug efflux, loss of PARP expression, HRR reactivation, replication fork stability, and feedback of the oncogenic signaling pathways. Therefore, overcoming PARP inhibitor resistance is a critical and complex challenge, and there is an urgent need to investigate the molecular mechanisms underlying this resistance in order to develop new therapeutic strategies. Our research aims to address the following questions: 1) Why do some cancers respond better to PARP inhibitors than others? 2) Why do certain patients initially respond to PARP inhibitors but eventually develop resistance? 3) What factors contribute to de novo resistance in some individuals with BRCA2 mutations?
To explore these questions, we employ CRISPR screening, RNA sequencing, and proteomic studies to identify the molecular mechanisms underlying PARP inhibitor resistance, using prostate cancer as a model system. Ultimately, we aim to identify specific vulnerabilities that can inform the development of novel combination therapeutic approaches to overcome PARP inhibitor resistance.
Project 3: Metabolic Reprogramming as a Master Driver of Lethal Cancer
There are currently no clinically effective methods to target the metabolic disruptions driving a subset of aggressive cancers. Fluorodeoxyglucose (FDG)-avid cancer lesions observed on positron emission tomography (PET) scans reflect tumor glycolytic activity and are linked to treatment resistance and poor outcomes. For years, we have identified metabolically reprogrammed aggressive cancers, including prostate cancer, using routine non-invasive FDG-PET imaging, yet we lack a comprehensive understanding of the biology behind these cancers and their associated therapeutic vulnerabilities. Consequently, there is limited insight into the molecular alterations driving these lesions and their connection to therapeutic responses.
The oncogenic activation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway in cancer cells reprograms cellular metabolism by enhancing the activity of nutrient transporters and metabolic enzymes. The PIK3R1 gene encodes the regulatory subunit of PI3K, and alterations or loss of PIK3R1 can increase the activity of PI3Kα and AKT. Through an analysis of 1,417 human prostate cancers from the Memorial Sloan Kettering Cancer Center (MSKCC) IMPACT cohort, we found that PIK3R1 was more frequently altered as in metastatic castration-resistant prostate cancer (mCRPC). Our experimental studies demonstrated that the loss of PIK3R1 in prostate cancer cell lines activates AKT, including insulin-induced phosphorylation of AKT, which promotes cell growth. We also retrospectively analyzed sequencing data from prostate cancer patients in the MSK-IMPACT cohort who had undergone FDG-PET/CT imaging. The FDG uptake was significantly higher in PIK3R1-altered prostate tumors (both primary and metastatic) compared to PIK3R1 wild-type tumors, suggesting that PIK3R1 alterations may define a distinct subtype of PI3K-dependent lethal prostate cancer and may be associated with high glucose uptake activity.
Our current study aims to elucidate the impact of the metabolic disruption caused by reprogrammed insulin and glucose metabolism due to PIK3R1 alterations on prostate cancer progression. Furthermore, we will evaluate novel therapeutic strategies that can exploit this metabolic reprogramming to reduce cancer progression and increase the survival of patients harboring mutations in PIK3R1.
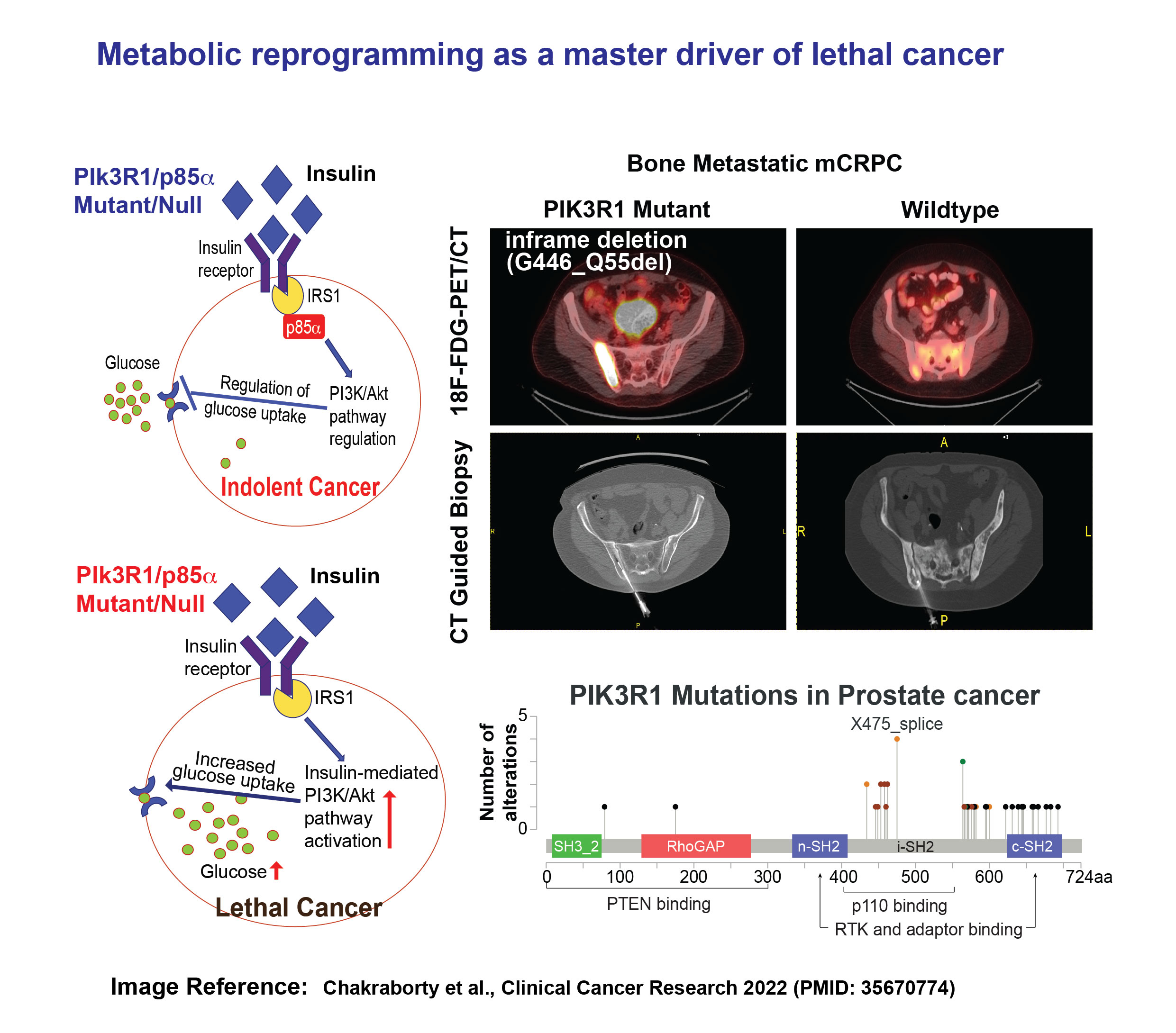
Project 4:
Impact of Y chromosome During Cancer Progression
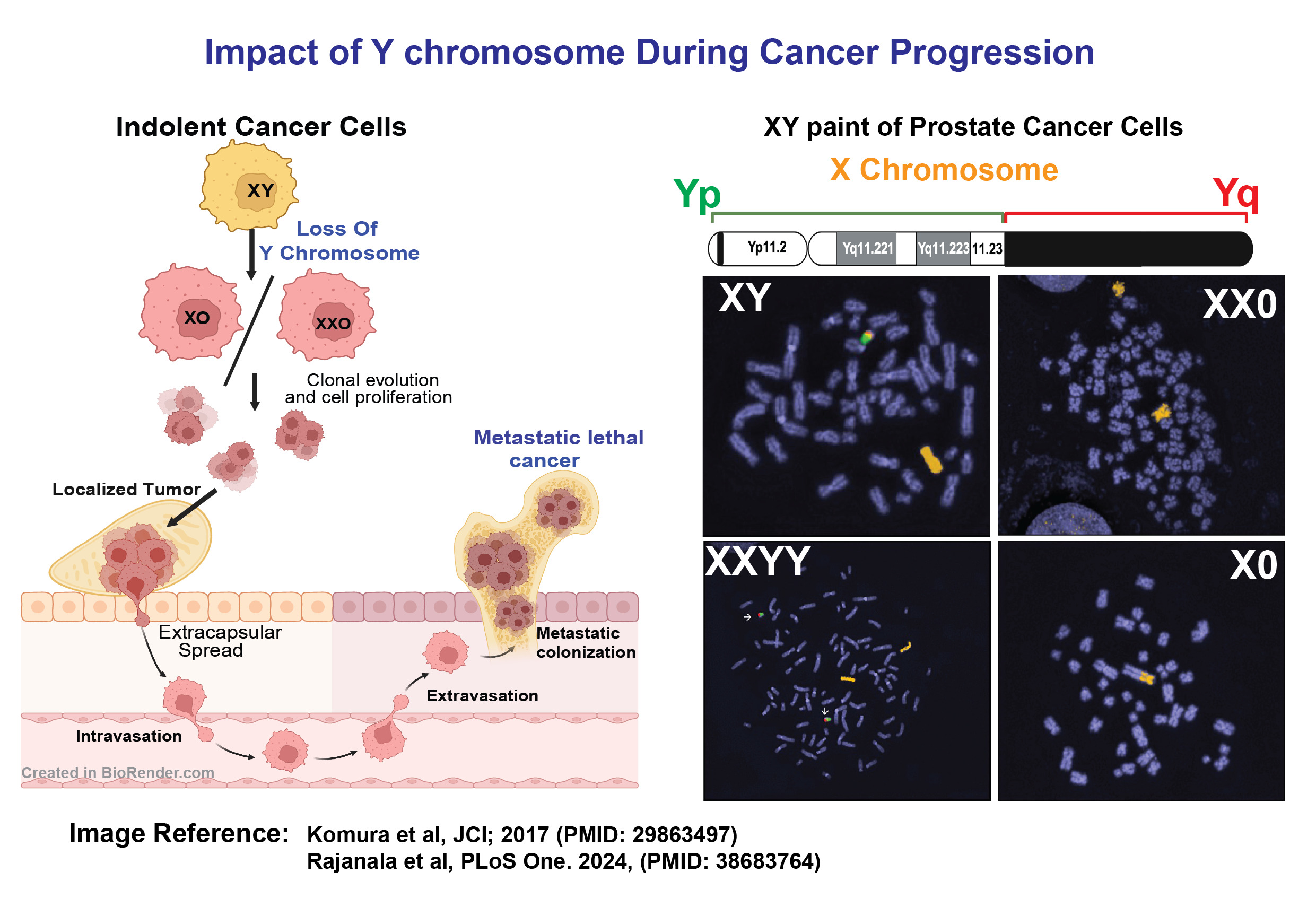
The incidence of cancer is significantly higher in men, with 50% being diagnosed in their lifetime compared to one-third of women. This disparity in cancer susceptibility and survival rates between genders may be attributed to a fundamental biological difference: the presence of the Y chromosome (ChrY) in males. Clinical evidence suggests that the loss of ChrY (LOY) plays a crucial role in gender-specific cancer occurrence and progression, particularly in genitourinary cancers, which are a serious global health issue affecting aging men the most. However, studying ChrY status in cancer cells is limited due to repetitive DNA sequences and low gene expression, leaving the impact of LOY and the role of ChrY genes in cancer progression largely unexplored.
The objective of our study is to investigate the role of various ChrY genes identified through CRISPR screening in the development of therapy-resistant prostate cancer. To achieve this, we will employ CRISPR to knock out individual ChrY genes and prospectively examine how these alterations induce resistance to androgen deprivation therapy. Additionally, we are establishing a CRISPR-Cas9 mediated method to completely eliminate ChrY from human genitourinary (prostate and bladder) cancer cells. Our research goal is to determine how the complete loss of ChrY affects tumor development and whether this deletion can lead to cancer metastasis.
Information on ChrY alterations has largely been overlooked or unreported in large cancer-specific genome-wide next-generation sequencing datasets due to challenges posed by the highly repetitive DNA sequences present in ChrY, combined with the low expression of ChrY genes in adults. Our aim is to address this knowledge gap by utilizing PacBio Single Molecule Real-Time (SMRT) long-read sequencing technology to analyze ChrY alterations in tumors from our comprehensive, continuously growing cohort of high-risk localized cancer patients. Therefore, the early identification of ChrY loss, even in localized disease, is crucial for developing improved treatment strategies, appropriate early risk stratification, and the potential avoidance of the healthcare cost burden and morbidity associated with overtreatment.
Project 5:
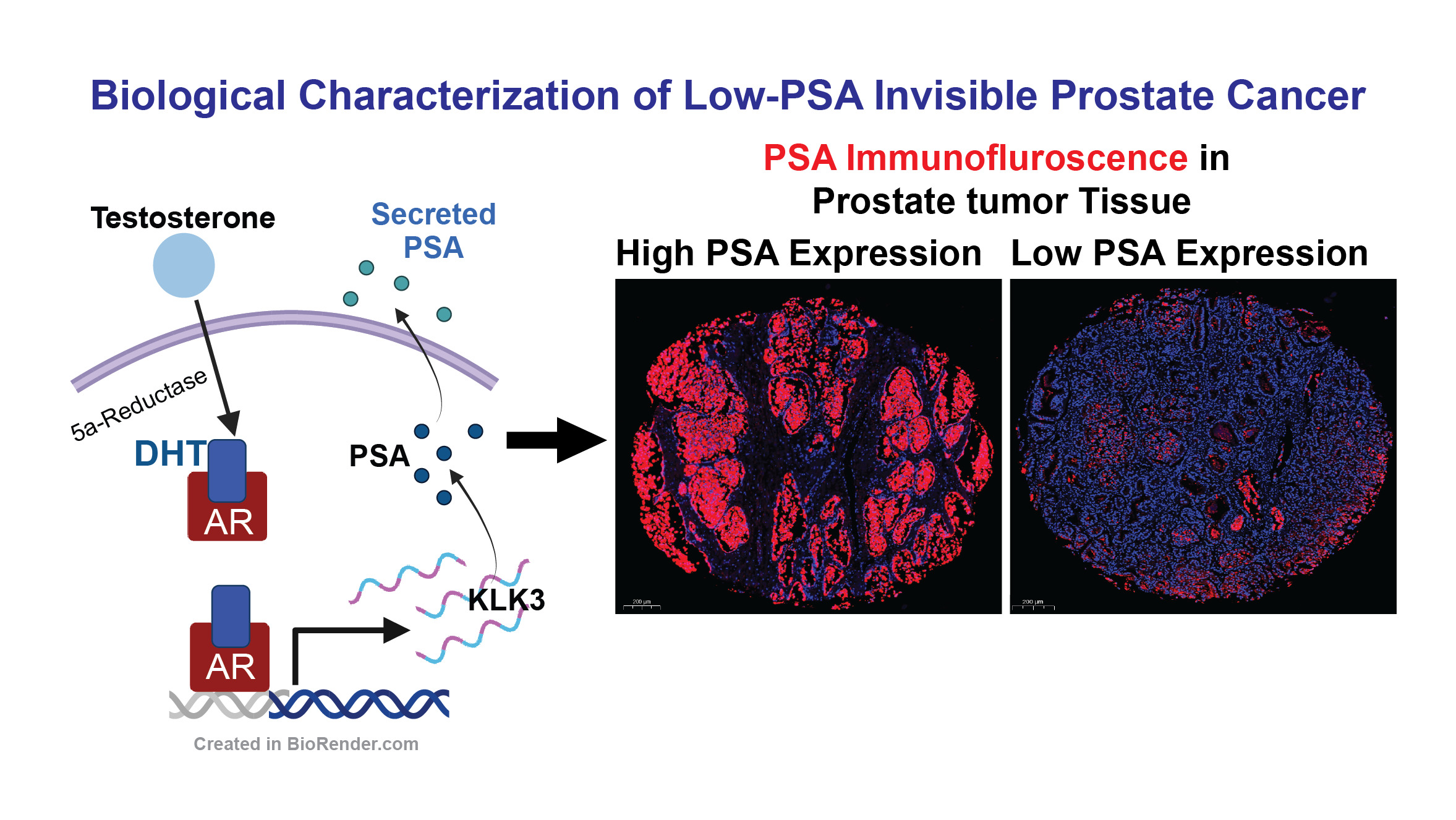
Biological Characterization of Low-PSA Invisible Prostate Cancer
Prostate-Specific Antigen (PSA) is a protein produced by the prostate gland, and its levels can be measured through a blood test to aid in the detection of prostate cancer. Elevated PSA levels can indicate the presence of prostate cancer, making PSA testing a widely used screening tool for early detection. Despite advances in molecular genetics and genome sequencing, distinguishing between lethal and non-lethal forms of prostate cancer in the early stages of the disease remains challenging.
A significant portion of prostate cancer patients have high-risk tumors that produce low levels of PSA. This characteristic is considered unfavorable, as it may indicate a lesser response to androgen deprivation therapy (ADT) and other androgen receptor-mediated drugs. Consequently, prostate cancer with low PSA secretion may represent a unique subtype of the disease that has not been fully characterized.
Our research identifies a distinct subtype of localized prostate cancer, demonstrating that low-PSA, high-risk tumors are significantly invasive and potentially lethal. This finding underscores the need for further investigation to better understand the biology of this poorly defined yet aggressive subtype of localized prostate cancer.
Project 6:
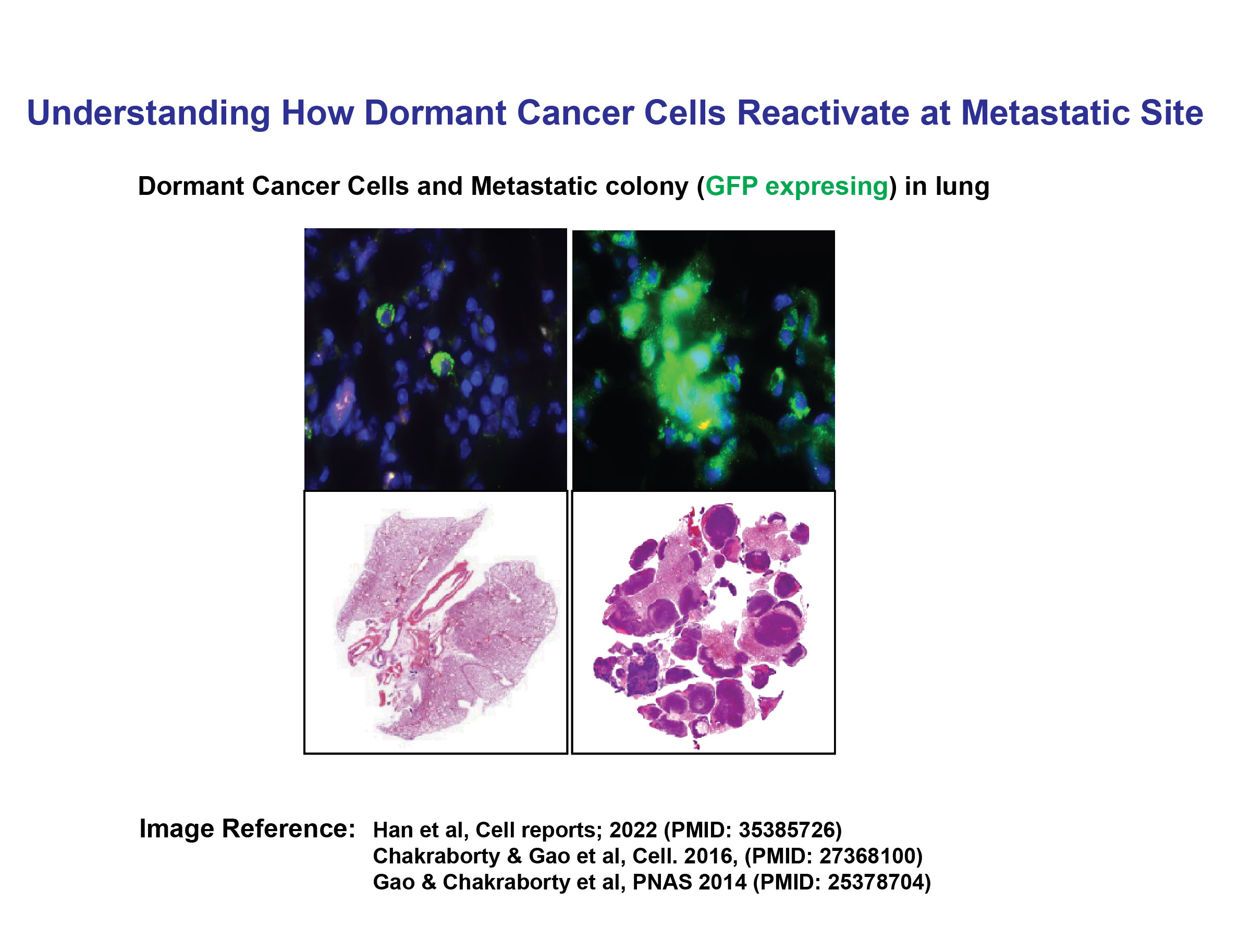
Understanding How Dormant Cancer Cells Reactivate at Metastatic Site
Cancer dormancy is a state in which cancer cells or microscopic tumors remain inactive for an extended period, evading detection and treatment. This phenomenon occurs when cancer cells survive initial therapies but enter a non-proliferative phase, often due to immune system control or an unfavorable microenvironment. Dormant cancer cells can persist in organs such as the bone marrow, lungs, or liver for years or even decades without causing symptoms. However, various factors—such as immune suppression, inflammation, hormonal changes, or alterations in the tumor microenvironment—can trigger their reactivation, leading to tumor recurrence or metastasis.
In our earlier study, we conducted whole-genome screening (including cDNA, shRNA, and miRNA approaches) and identified several novel metastasis-related genes, characterizing their roles in cancer dormancy and metastasis. These studies demonstrated that interactions between tumor cells and their microenvironment play a crucial role in the metastatic reactivation of breast cancer cells at metastatic sites, leading us to identify potential novel therapeutic targets.
Therefore, understanding the mechanisms underlying cancer dormancy and reactivation is essential for developing targeted therapies to prevent relapse and improve long-term patient outcomes.

