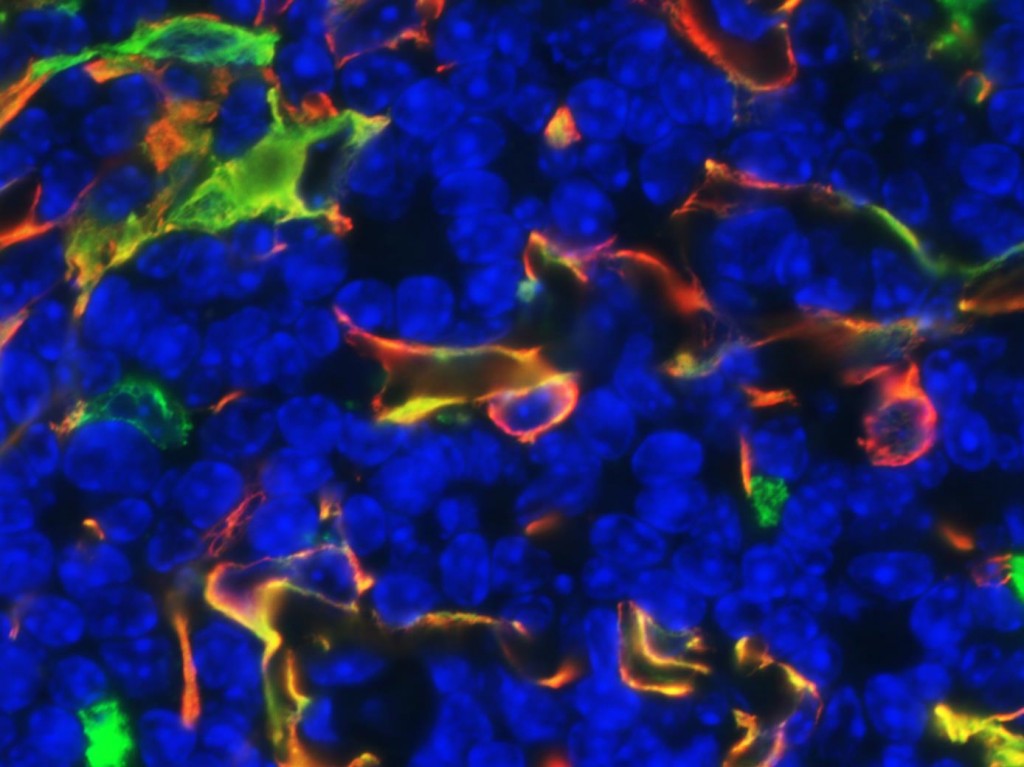
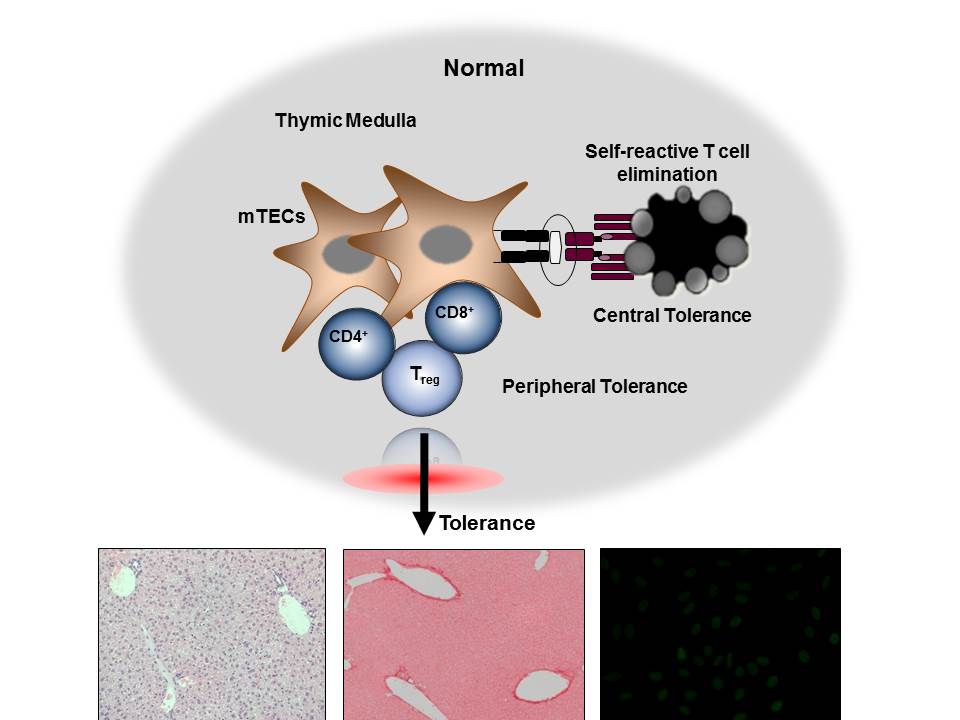
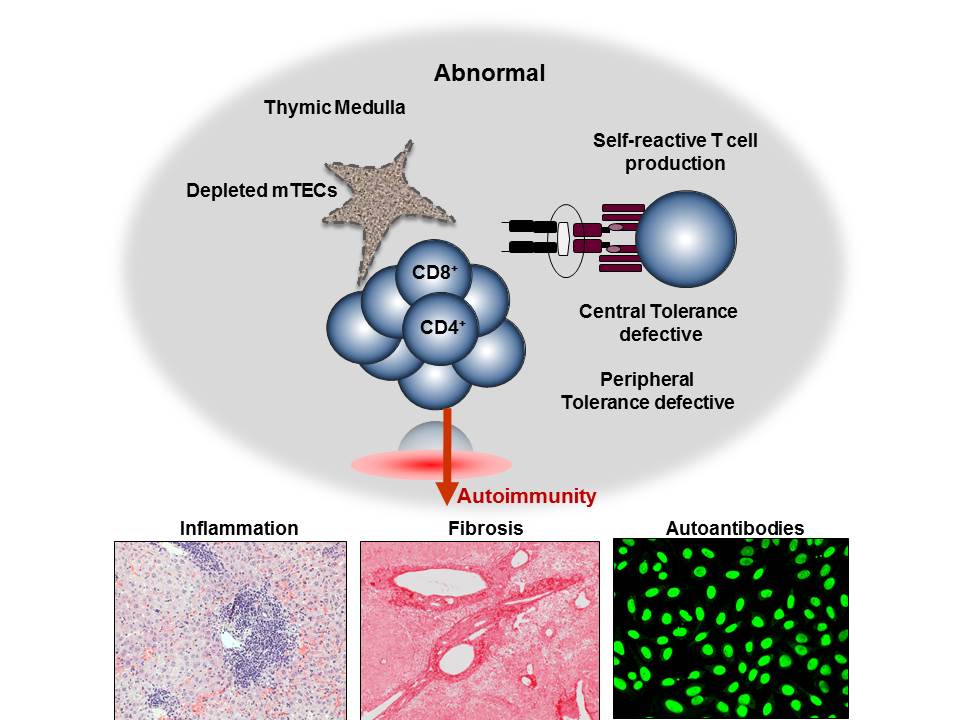
Research in the laboratory concentrates on elucidating the mechanisms that regulate T cell-mediated autoimmunity. We are using genetically engineered mice lacking a specific cell population in the thymus to disrupt the normal development of T cells and determine whether/how this disruption results in the development of autoimmunity. T cells originate from bone marrow precursors that migrate to the thymus where they undergo a series of differentiation steps. Stromal thymic epithelial cells (TECs) regulate the survival and maturation of immature thymocytes through presentation of self-antigens on their surface. Medullary thymic epithelial cells (mTECs) in particular regulate the elimination of self-reactive T cells, a process called central tolerance. In addition, mTECs regulate the production of T regulatory (Treg) cells which suppress peripherally activated T cells (peripheral tolerance). Disruption of interactions between mTECs and T cells through mTEC depletion, leads to aberrant elimination of autoreactive T cells, defective central and peripheral tolerance and organ-specific autoimmunity, manifested as T cell-containing inflammatory infiltrates and autoantibody production. Our effort is currently focused on understanding how disruption of thymic cross-talk between the medullary epithelium and T cells affects antigen presentation and T cell selection in the thymus, as well as the type of autoimmune responses generated against the peripheral tissues of these mice.