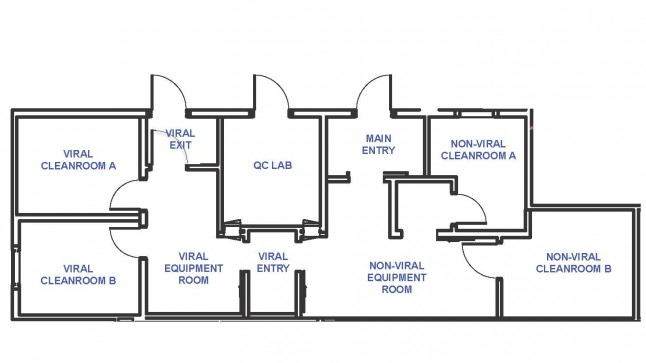
The VCTL occupies over 1,200 square feet of space on the 5th floor of the Leon and Norma Hess Center for Science and Medicine. It provides a dedicated, controlled space to manufacture human therapeutics for Phase I and II clinical studies in accordance with current Good Manufacturing Practice (cGMP) and compliant with FDA regulations (CFR 21 Part 11, 210, and 211), that will ensure the safety, identity, purity and potency of the manufactured products.
VCTL operations are guided by over 100 Standard Operating Procedures (SOP), Master Batch Records (MBR), and Standard Forms (SF) documents that ensure adherence to applicable regulatory requirements and cGMP standards for manufacturing quality including facilities, equipment, personnel training, and work performance. A dedicated and independent Quality Assurance Specialist ensures compliance by conducting audits, inspection, and reviews and reports any issues to the Director of the VCTL.
The VCTL completed execution of a validation master plan prior to start of operations which includes over 50 Installation Qualification (IQ), Operation Qualification (OQ), and Performance Qualification (PQ) validation protocols for facility, equipment, and utilities.